Docket: T-1632-16
Citation: 2020 FC 814
Ottawa, Ontario, September 10, 2020
PRESENT: The Honourable Madam Justice St-Louis
BETWEEN:
ELI LILLY CANADA INC., ELI LILLY AND COMPANY, LILLY DEL
CARIBE INC., LILLY, S.A. and ICOS CORPORATION INC.
Plaintiffs/Defendants by counterclaim
and
APOTEX INC.
Defendant/Plaintiff by counterclaim
PUBLIC JUDGMENT AND REASONS
(Confidential Judgment and Reasons issued on August 6, 2020)
III. The pleadings and the results
V. The statutory scheme under which the matter proceeds
(2) Dr. Robert Michael Williams
A. Relevant date for claim construction
(2) One construction for all purposes
(3) Purposive construction: essential and non-essential elements
(4) Purposive construction: the patentee’s words
F. Claims needing construction
(2) Construction of Claims 1, 3-4
(b) Claim 1 first paragraph: “a desired cis-diastereomer” versus “the desired cis-diastereomer”
(d) Claim 1 step c: phase separation vs separation from the mixture
(e) Essential elements of Claims 1, 3-4
(3) Construction of Claims 7, 8-10
(c) Essential elements of Claims 7 and 8-10
(b) Claim 12 step a and step b: the variants
(d) Essential elements of Claim 12
VI. Apotex’s counterclaim of invalidity
(1) The anticipation allegations
(2) The anticipation framework
(a) Section 28.2 of the Patent Act and the Sanofi test
(b) The disclosure requirement
(i) Claim 1 step a (referenced in Claims 3 and 4)
(ii) Claim 1 step b (referenced in Claims 3 and 4)
(iii) Claim 1 step c (referenced in Claims 3 and 4)
(c) The enablement requirement
(3) Conclusion on anticipation
(1) The obviousness allegations
(a) Section 28.3 of the Patent Act
(b) The Sanofi test on obviousness
(c) First step: identify the notional PSA and the relevant common general knowledge of that person
(ii) 1986: the Beloit framework
(iii) Section 28.3 of the Patent Act
(vi) The meaning of the term inventive concept
(vii) The subject-matter defined by a claim of the 540 Patent
Claim 7 step d and Claims 8–10
VII. Lilly’s claim of infringement
C. Apotex process: ||||||||||||||||||||
D. Apotex process : ||||||||||||||||||||||||||||
E. Apotex process: ||||||||||||||||||
VIII. Election between damages and accounting of profits
IX. Declaratory relief, injunction relief and/or delivery up.
I.
Introduction
[1]
These additional reasons relate to an action by the Plaintiffs (hereinafter collectively referred to as “Lilly”) against Apotex Inc. (Apotex) and a related counterclaim by Apotex, in regards to Canadian Patent No. 2,492,540 Patent [the 540 Patent].
[2]
The reasons relating to the Plaintiffs’ actions and related counterclaims, in regards to Canadian Patent No. 2,371,684 Patent [the 684 Patent] are exposed in case docket T-1627-16 and will be placed on this file (Eli Lilly Canada Inc. and als. v Mylan Pharmaceuticals ULC, 2020 FC 816).
[3]
Hence, these additional reasons are concerned with the validity and infringement, at the liability phase, of the 540 Patent, entitled “Modified Pictet-Spengler Reaction and Products Prepared Therefrom”
.
II.
Procedural background
[4]
Lilly initially sued Apotex, Mylan Pharmaceuticals ULC, Teva Canada Limited, and Pharmascience Inc.-Laboratoire Riva Inc. in independent actions for infringement of patents related to tadalafil. Each of the Defendants denied infringement and counterclaimed for a declaration of invalidity of the patents asserted against them. Over the course of these proceedings, Lilly has asserted four patents against the four Defendants: (1) the 684 Patent, which expired on April 26, 2020, and relates to a dosage form of tadalafil; (2) the 2,379,948 Patent, which expired on April 26, 2020, and relates to a formulation comprising tadalafil; (3) the 540 Patent, which will expire on July 14, 2023, and relates to a manufacture process for making tadalafil; and (4) the 2,226,784 Patent [the 784 Patent] which expired on July 11, 2016, and relates to the use of tadalafil to treat ED.
[5]
On September 8, 2017, Prothonotary Tabib, at the request of the parties, bifurcated the actions as between liability and quantification phases. As per Prothonotary Tabib’s Order, this liability phase addresses the following issues: (i) whether the patents have been infringed by the Defendants; ii) whether the patents are valid; (iii) except for paragraphs 9, 28-36, 37-42 and 175 of Apotex’s Amended Statement of Defence and Counterclaim which shall be addressed in the quatification phase, whether Lilly are entitled to declaratory relief, injunctive relief, and delivery up; and (iv) Lilly’s entitlement, if any, to elect as between damages and an accounting of profits (except as it relates to paragraphs 28-36 of the Defence).
[6]
On July 3, 2019, Prothonotary Tabib granted Lilly leave to amend their Statement of Claims, whereby only claims for infringement of the 684 Patent against all Defendants, and claims for infringement of the 540 Patent against Teva, which was subsequently withdrawn, and Apotex were maintained.
[7]
Prothonotary Tabib also then granted Lilly leave to add, against all Defendants, claims for the infringement of the 784 Patent by reason of the manufacturing, importing and stockpiling of tadalafil for ED prior to the expiration of the 784 Patent, and springboard damages flowing for that infringement. As a condition for granting leave to amend, all issues of validity, infringement and quantification relating to the 784 Patent, were bifurcated and will be the subject of a separate trial after the determination of the liability issues for the 684 and 540 Patents.
[8]
Although the actions have not been consolidated, they have been case managed together, and proceeded together to trial for the liability phase, with hearings conducted from December 5, 2019 to February 7, 2020. Although the actions regarding the two patents at issue have not been bifurcated, the parties all agreed to the trial being divided in two separate components, the first pertaining to the liability phase of the 684 Patent, which involves all four Defendants, and the other pertaining to the liability phase of the 540 Patent, which involves only Apotex as the Defendant.
[9]
I am thus providing here additional reasons pertaining solely to the litigation between Lilly and Apotex in regards to the 540 Patent. Certain passages of the reasons pertaining to the 684 Patent are repeated in these additional reasons, with the risk of redundancy, in order to allow for a reading of these additional reasons on a stand-alone basis.
[10]
The parties have not disputed that the law is the same in both components of the trial, although surprisingly, and as I will outline in the discussion regarding anticipation, Lilly presented different versions of the principle guiding the disclosure requirement of the anticipation analysis in each component of the trial.
III.
The pleadings and the results
[11]
The Plaintiffs in this action are Eli Lilly Canada Inc., Eli Lilly and Company, Lilly Del Caribe, Inc., Lilly, S.A. and ICOS Corporation Inc. Apotex Inc. is the lone Defendant.
[12]
Eli Lilly Canada Inc. has a principal place of business in Toronto, Ontario. Eli Lilly and Company has a principal place of business in Indianapolis, Indiana. Lilly Del Caribe, Inc. has a principal place of business in Caroline, Puerto Rico, and is incorporated in the Cayman Islands. Lilly, S.A. has a principal place of business in Madrid, Spain. ICOS Corporation Inc. has a principal place of business in Indianapolis, Indiana. Apotex Inc. is a generic drug maker based in Toronto, Ontario.
[13]
Apotex has four, still current, regulatory approved processes to make tadalafil via two main suppliers, both based in India: |||| and ||||||||||||||||||||||||. |||| procured the intermediaries from |||||||||||||||||||||||||||||||||||||||| in China, |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| in India, and |||||||||||||||||||||| in China, which sourced it from |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| | in China. ||||||||||||||||||||||||, on the other hand, made the whole of the tadalafil itself.
[14]
Lilly assert that Apotex’s four regulatory approved processes to make tadalafil infringes Claims 1, 3–4, 7–10, 12 of the 540 Patent (the asserted Claims).
[15]
Lilly rely upon the statutory presumption as set out in section 55.1 of the Patent Act, RSC 1985, c P-4 [the Patent Act], and on the common law presumption (Hoffmann-La Roche Ltd v Apotex Inc, 1983 CarswellOnt 871 at paras 23-25 (ONHC) [Hoffmann], aff’d 1984 CarswellOnt 1197 (ONCA)), for the |||||||||||||||||||||| process, to argue that Apotex bears the burden to prove that it did not infringe the asserted Claims.
[16]
Lilly consequently seek a declaration that Apotex infringed or induced infringement of the asserted Claims of the 540 Patent, a declaration that they are entitled to elect between damages and an accounting of profits, an order that they are entitled to a declaratory relief, injunctive relief and/or delivery up, and costs.
[17]
Apotex denies infringement and initially raised the Gillette defence (Free World Trust v Electro Santé Inc, 2000 SCC 66 [Free World Trust]). Against the statutory presumption raised by Lilly, Apotex answers that it is not applicable because tadalafil is not a “new product”
since tadalafil and its intermediaries were subject to previous patents. Against the common law presumption raised by Lilly, Apotex answers that the presumption has never been applied, not even in Hoffmann where it was enunciated in obiter dictum, that the facts of the Hoffmann case are extreme, and should be distinguished primarily on the basis that Apotex duly cooperated to disclose all information it had on the processes.
[18]
Apotex also responds that none of its processes infringed the 540 Patent. It argues that the |||||||||| process does not have a |||||||||||||||||||||||||||||||||||||||||| with acetic acid step, which involves the construction of Claim 7, nor does it use isopropyl alcohol as a solvent for the Pictet-Spengler reaction (PSR), using instead ||||||||||, which involves the construction of Claim 12. It adds that the |||||||||||||||||||||||| process does not have a |||||||||||||||||||||||||| step after the PSR, which involves the construction of Claim 1, does not have a |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| || step, which involves the construction of Claim 7, and uses |||||||||||||| for the PSR rather than isopropyl alcohol, which involves the construction of Claim 12. The Court’s claims construction will essentially determine the issues for these two processes.
[19]
Apotex argues that the |||||||||||||||||||||||||||||||| process turns on the issue of regulatory exemption to infringement of section 55.2 of the Patent Act, while the |||||||||||||||||||||| process turns on Lilly’s evidentiary burden to prove the processes described in the batch record and regulatory submissions are fabricated, as well as the applicability of the two presumptions of infringement.
[20]
Apotex seeks a declaration that the 540 Patent or the asserted Claims are invalid, as well as costs.
[21]
On January 6, 2020, the parties have jointly outlined the issues as follows:
a) Construction of Claims 1, 3–4, 7–10, 12 of the 540 Patent;
b) Whether any of the asserted Claims are infringed;
c) Whether the Gillette Defence applies;
d) Whether the asserted Claims are invalid by reason of :
i. Anticipation: does Canadian Patent Application No 2,412,594 (the 594 Application, known as the Gellibert Application) anticipate the subject-matter of Claims 1, 3-4 of the 540 Patent?
ii. Obviousness: would the subject-matter defined by the asserted Claims of the 540 Patent have been obvious on the Claim date to a person skilled in the art?
iii. Lack of sound prediction/no demonstration utility: have the requirements of either demonstration or sound prediction of utility as of the filing date of the 540 Patent met?
iv. Overbreadth: are the asserted Claims of the 540 Patent broader than either the invention made by the named inventor of the 540 Patent or the invention disclosed in the specification of the 540 Patent?
v. Inutility/inoperability: does the subject-matter defined by the asserted Claims of the 540 Patent in fact possess utility?
vi. Insufficiency: does the 540 Patent satisfy the requirements of subsection 27(3) of the Patent Act?
e) Whether the Plaintiffs are entitled to elect as between damages and an accounting of profits;
f) Whether the Plaintiffs are entitled to declaratory relief, injunctive relief and/or delivery up.
[22]
At closing, Apotex did not assert the lack of sound prediction/no demonstrated utility, insufficiency, nor did it assert the Gillette Defence. Hence, the remaining grounds of invalidity raised by Apotex are those of anticipation, obviousness, overbreadth and inutility/inoperability.
[23]
The Court must adjudicate the issues regarding the type and entitlement to reliefs if necessary.
[24]
In brief, and for the reasons exposed below, I find Claims 1, 3–4, 7–10, 12 of the 540 Patent are invalid as Claims 1, 3–4 are anticipated, and all asserted Claims are obvious.
[25]
However, if I were wrong and the asserted Claims were valid, I find Claims 1, 3–4 to be infringed by Apotex’s |||||||||| process, while Claims 7–10, 12 of the 540 Patent not to be infringed by Apotex.
IV.
Tadalafil
[26]
The drug substance at the heart of these proceedings is tadalafil, used, among other things, to treat male erectile dysfunction (MED). In this regard, tadalafil is the active pharmaceutical ingredient (API) of the drug product marketed by Lilly under the brand name CIALIS, and by Apotex under the brand name Apo-Tadalafil.
[27]
Tadalafil is known as a phosphodiesterase (PDE) 5 inhibitor. The first approved PDE-5 inhibiter was sildenafil, commercialised by Pfizer under the brand name Viagra, and approved in Canada on March 9, 1999. Tadalafil is the second in class PDE-5 inhibiter drug product and both have had considerable commercial success.
[28]
In brief, tadalafil works to promote the relaxation of the penis’ smooth muscle, which somewhat counterintuitively for a layperson, promotes penile erection. In brief, the penis’ smooth muscle, known as the corpora cavernosa, is in a contracted state when in normal resting state, and so restricts the arteries supplying blood to the penis. When an erection is triggered, the smooth muscle relaxes, no longer restricts the supply of arterial blood, which causes the penis to become tumescent. The smooth muscle relaxation results from a cascade of complex biochemical reactions within the body. Normally, sexual stimulation triggers the release of nitric oxide, which in turn leads to an increase in the production of a molecule called cyclic guanosine-3-5 monophosphate (cGMP). This cGMP molecule regulates the activity of other intracellular proteins and leads to the relaxation of the smooth muscle. Increasing cGMP promotes smooth muscle relaxation, which promotes penile erection. The intracellular breakdown of the cGMP is regulated by a class of enzymes known as cyclic nucleotide PDE, and in the penis, the most prevalent is the PDE-5 family. Inhibiting PDE-5 results in a slower breakdown of cGMP, which then accumulates, promotes the relaxation of the smooth muscle and, in turn, penile erection.
[29]
Tadalafil was first claimed in the British patent GB no 9401090.7 (which Canadian equivalent is the 2,181,377 Patent (the 377 Patent)), filed on January 21, 1994 by Laboratoires Glaxo. A number of other patents were also granted in relation to tadalafil, now owned by Lilly as the results of successive commercial transactions.
[30]
The 540 Patent relates to a commercial manufacturing process to synthesize tadalafil, and bears a particular focus on the synthesis of the key intermediate compound, a cis-diastereomer having the R,R absolute stereochemistry known as cis-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester. The synthesis of this key intermediate is achieved by carrying what is described as an improved PSR in which the desired cis-diastereomer is insoluble at reflux temperature or lower, and the undesired trans-diastereomer is soluble at reflux temperature or lower, resulting in concomitant crystallisation, and separation, of the desired cis-diastereomer product.
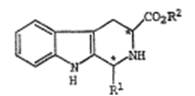
[31]
The PSR reaction was discovered in 1911. Named after its discoverers, it is a chemical reaction in which a β-arylethylamine undergoes condensation with an aldehyde or ketone followed by a ring closure. In the patent at bar, it is a method of attaching a new six membered ring to an existing ring system. Another concept, the “crystallization-induced asymmetric transformation”
(CIAT) relates here to the in situ transformation of trans-diastereomer to cis-diastereomer, driven by the crystallizations of the desired cis-diastereomer in the mixture (equilibration), resulting in high yield, high purity and faster processing times with fewer steps.
V.
The statutory scheme under which the matter proceeds
[32]
The parties agree that the law of patents is wholly statutory. The Supreme Court of Canada (SCC) has confirmed it again in 2008, in one of the landmark decision I will discuss later, Apotex v Sanofi-Synthelabo Canada 2008 CSC 61[Sanofi]. The SCC cited Justice Judson’s words in Commissionner of Patents v Farbwerke Hoechest Aktiengesellschaft Vormals Meister Lucius & Bruning, [1964] SCR 49 at 57 that “There is no inherent common law right to a patent. An inventor gets his patent according to the terms of the Patent Act, no more and no less”
(Sanofi para 12). The SCC also cited Lord Walker’s words in Synthon B.V. v SmithKline Beecham plc, [2006] 1 All E.R. 685, [2005] UKHL 59, at paras 57-58:
57. The law of patents is wholly statutory, and has a surprisingly long history… In the interpretation and application of patent statutes judge-made doctrine has over the years done much to clarify the abstract generalities of the statutes and to secure uniformity in their application.
58. Nevertheless it is salutary to be reminded, from time to time, that the general concepts which are the common currency of patent lawyers are founded on a statutory text, and cannot have any other firm foundation (Sanofi at para12).
[33]
As the patent in suit was filed after October 1, 1989, the current provisions of the Patent Act apply. The relevant sections of the Patent Act are reproduced in Annex II for ease of reading.
I.
Burden of proof
A.
Infringement
(1)
General
[34]
To establish infringement, Lilly must prove, on a balance of probabilities, that the processes used by Apotex’s suppliers include all of the essential elements of one or more claims of the 540 Patent. In fact, “there is no infringement if an essential element is different or omitted”
but “there may still be infringement, however, if non-essential elements are substituted or omitted”
(Free World Trust at para 31).
[35]
However, the patentee’s burden of proof can shift on the alleged infringer under a statutory and a common law presumption. Lilly submit that both presumptions apply here.
(2)
Statutory presumption
[36]
The statutory presumption is stated at section 55.1 of the Patent Act: “In an action for infringement of a patent granted for a process for obtaining a new product, any product that is the same as the new product shall, in the absence of proof to the contrary, be considered to have been produced by the patented process”
(my emphasis).
[37]
Lilly urge the Court to interpret the term “new product”
of section 55.1 in a way akin to the term “new drug”
, defined in section C.08.001 of the Food and Drug Regulations, CRC c 870 [Food and Drug Regulations]. Lilly thus assert that CIALIS (and their other tadalafil product ADCIRCA) are “new products”
, and that section 55.1 of the Patent Act therefore applies to reverse the burden on infringement. However, Lilly do not detail their position, accepting that the Court’s decision in Eli Lilly and Company v Apotex Inc, 2009 FC 991 [Cefaclor], aff’d 2010 FCA 240 may be subject to comity. They confirm merely seeking to preserve their rights on appeal in this regard.
[38]
Apotex responds that section 55.1 of the Patent Act does not apply here because tadalafil is not a “new product”
: processes to make tadalafil have been the subjects of prior patents, and tadalafil was known prior to the filing of the 540 Patent. Apotex stresses that Lilly’s proposed interpretation of “new product”
, akin to the term “new drug”
found in the Food and Drug Regulations — hence as any product that has not been sold on the market — was specifically rejected in Cefaclor at para 214. In Cefaclor, Lilly had argued that the word “product”
(used in the current section 55.1 of the Patent Act), replaced the word “substance”
(used in former subsection 39(2) of the Patent Act), as a result of an amendment in 1993 in order to give effect to para 1709(11)(a) of the North American Free Trade Agreement Between the Government of Canada, the Government of Mexico and the Government of the United States, 17 December 1992, Can TS 1994 No 2. They had submitted that it meant a “product that has not been sold on the market before”
, but Justice Gauthier, now at the Federal Court of Appeal (FCA), did not accept this argument.
[39]
Apotex also points to the decision of the Court in Merck & Co Inc v Apotex Inc, 2010 FC 1265 at paras 134-186, aff’d 2011 FCA 363, where Justice Snider interpreted the word “new”
in the term “new substance”
of former section 39(2) of the Patent Act, and found it to mean “not previously known”
. The FCA neither condemned nor endorsed her interpretation.
[40]
Despite Lilly not detailing their position on the interpretation of the term “new drug”
from the Food and Drug Regulations, I note that these regulations (s C.08.001) define the term, in paragraph c, as a drug that “(…) has not been sold for that use or condition of use in Canada (..)”
, precisely the interpretation rejected by Justice Gauthier in Cefaclor.
[41]
Judicial comity requires that I follow an earlier decision unless I am persuaded that it was wrongly decided. However, rather than putting forth further arguments, Lilly accepted that the Court may be subject to comity, and confirmed that, before this Court, they merely sought to preserve their rights on appeal. They have therefore not convinced me that Cefaclor is wrong. Since Lilly have not demonstrated tadalafil is a “new product”
in regards the statutory presumption of section 55.1 of the Patent Act, they retain the evidentiary burden to prove infringement.
(3)
Common law presumption
[42]
Lilly raise the common law presumption in regards to the |||||||||||||||||||||| process. They argue that the set of circumstances surrounding this process justifies applying the common law presumption enunciated in Hoffmann at para 23: “when the subject-matter of the allegation lies particularly within the knowledge of one of the parties, that party must prove it, whether it be an affirmative or negative character”
. Lilly argue that the common law presumption applies to the |||||||||||||||||||||| process Apotex is using to make tadalafil for sale in Canada as it is uniquely within its purview.
[43]
Lilly point to their expert witness, Dr. Trevor Laird’s uncontested testimony that he does not believe that high quality compound can be produced by the process described in the batch record of ||||||||||||||||||||||, thus suspecting the falsification of the batch record. For Lilly, the batch record should thus not be given any weight, and since Apotex has not produced any documents on this issue, the burden should shift from Lilly being required to prove infringement to Apotex being required to prove non-infringement, which it has not done. Since only Apotex can obtain proper records of the process, Lilly allege that this situation falls squarely within the ambits of the presumption. Insisting that Apotex’s situation is unique because it dealt with ||||, which dealt with ||||||||||||||||||||||, which sourced the intermediate from ||||, Lilly cite Eli Lilly & Co v Apotex Inc, 2000 CarswellNat 185 (FCTD), to argue that it is proper to require Apotex to make such a request for information. Lilly also argue that Apotex presented no evidence that it advised its suppliers not to infringe the 540 Patent. To counter Apotex’s argument that they have not taken enough steps to seek out additional information regarding the |||||||||||||||||||||| process, Lilly answer that they cannot trust Apotex to provide true documents when the batch record first provided is falsified, and that the discontinuance of ||||||||||||||||||||||’s business meant that it would not have been possible to obtain further records. As I will outline later in these reasons, Mr. Ramandeen Singh Bagga testified before the Court as a fact witness for Apotex, as its VP Global Direct Procurement. Lilly somewhat acknowledge not having further cross-examined Mr. Bagga on the |||||||||||||||||||||| process, but raise the fact that Mr. Bagga testified even Apotex was not able to obtain any further information from this company. Lilly therefore argue that it is unclear how Mr. Bagga could have given any testimony that was not impermissible hearsay.
[44]
Apotex mainly argues the Hoffmann decision setting out the common law presumption has never been applied, and should be circumscribed to its facts, where the defendant was a licensee under the plaintiffs’ patent and had instructed its supplier not to divulge any information regarding its process to the plaintiffs or their lawyer. Apotex again points to the Cefaclor decision in which Justice Gauthier clarified the applicability of the Hoffmann common law presumption. Apotex essentially argues that (1) it diligently sought out the requested information by providing numerous documents regarding the |||||||||||||||||||||| process and seeking others, as Mr. Bagga testified; (2) Lilly did not even attempt to compel evidence from Apotex; and (3) Lilly put forward an extract of Apotex’s Abbreviated New Drug Submission (ANDS), a regulatory document, as a business record, without attempting to prove on a balance of probabilities this document is also falsified. This ANDS extract also outlines the |||||||||||||||||||| process.
[45]
As Justice Gauthier did in Cefaclor at paras 219–223, I also find the evidence adduced in these proceedings does not allow me to conclude that Apotex did not diligently seek to provide the requested process documents, nor that Lilly diligently sought further information from Apotex. Lilly’s argument regarding precisions that could have been sought from Mr. Bagga in cross remains unconvincing. Furthermore, I note that Lilly did have information on the process through the extract of Apotex’s ANDS, and that Dr. Laird considered this document at paragraph 83 of his Infringement Expert Report. Lilly did not explain how this regulatory document could contain fabricated information despite not being flagged by the regulatory agency, and they have not met the burden to show that it contains a fabricated process. The presumption does not apply and the burden to prove infringement remains Lilly’s.
B.
Invalidity
[46]
Under subsection 43(2) of the Patent Act, after the patent is issued, it shall, in the absence of any evidence to the contrary, be valid. The statute thus creates a presumption of the patent’s validity, and the burden is on Apotex to prove, on a balance of probabilities, that the patent is invalid (Whirlpool Corp v Camco Inc 2000 SCC 67 [Whirlpool] at para 75)
II.
Facts witnesses
A.
Lilly’s facts witnesses
(1)
Dr. Michael Martinelli
[47]
Dr. Martinelli is one of the listed inventors of the 540 Patent. He holds a BSc from the State University of New-York, a PhD from Wesleyan University in natural product synthesis, and a Post-Doctorate fellowship from Harvard.
[48]
Working at Lilly from 1987 until 2003, Dr. Martinelli was involved in the due diligence of ICOS, in anticipation of a joint venture between Lilly and ICOS in 1998. He testified regarding how he and Mr. Joseph Matthew Pawlak worked to find a better process to make tadalafil due to numerous shortcoming of the processes used at ||||, ||||||, and ||||||||. He remembered asking Mr. Pawlak, in August 1998, to replicate the processes at |||||| and ||||||||. ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||.
[49]
Dr. Martinelli was a credible witness, although he had little recollection about the specific steps he executed some 20 years ago. He relied heavily on the still existing paper trail of documents and, at trial, adjusted some aspects of his testimony given on discovery, having read Mr. Pawlak’s notebook before testifying in Court. He testified having used 2–3 notebooks per year himself, although only a blank notebook was located by Lilly during discovery.
(2)
Mr. Joseph Matthew Pawlak
[50]
Mr. Pawlak is also a listed inventor of the 540 Patent. After obtaining a BSc in chemistry, he worked in a sub factory synthesizing intermediate compounds before joining Lilly in August 1997. He left the company in 2015. Working under Dr. Martinelli, he was the principal investigator of the tadalafil synthesis process at Lilly.
[51]
Similar to Dr. Martinelli, Mr. Pawlak testified about Lilly’s invention story, albeit in greater details about notably the solvents, the acids, and the reaction conditions that were tried for the PSR.
[52]
Mr. Pawlak’s was credible, and relied on his notebook.
B.
Apotex’s fact witness
(1)
Mr. Ramandeen Singh Bagga
[53]
Mr. Bagga testified before the Court as the VP Global Direct Procurement of Apotex. After obtaining a BSc in Pharmacy and a MBA, he worked mainly in sales for multiple Indian pharmaceutical companies, including ||||||||||, before joining Apotex in January 2011 as the VP Business Development and Marketing and Sales. In 2015, he became the VP Global Supply Chain Management at Apotex.
[54]
Mr. Bagga testified primarily on how and where Apotex sourced the APIs, on the general content of an open or closed part of a drug master file (DMF), on the content of an ANDS, and on the measures taken by Apotex to provide Lilly accurate process documents of its suppliers.
[55]
Mr. Bagga was a credible witness, answering in a forthright manner.
III.
Expert witnesses
A.
Lilly’s expert witness
(1)
Dr. Trevor Laird
[56]
Dr. Laird holds a BSc (chemistry) degree from the Imperial College of London, and a PhD in organic chemistry from the London University. Before becoming a consultant, he was in charge of chemists for SmithKline. He was qualified as an expert in synthetic organic chemistry.
[57]
Dr. Laird was Lilly’s lone expert witness, and he opined on claim construction, infringement, and validity. He signed a Construction Expert Report on August 30, 2019, an Infringement Expert Report on August 30, 2019, a Validity Expert Report on January 7, 2020, and a Reply Expert Report on January 7, 2020 (exhibits 116, 117, 118, and 119 respectively).
[58]
Dr. Laird’s opinion should be approached with caution. I have no doubt as to his qualifications obviously, but he appeared result-oriented in his claim construction. There were inconsistencies in the ways he construed the claims, namely by suggesting equivalents for some elements but not for others, without providing proper justifications.
B.
Apotex’s expert witnesses
(1)
Dr. Neil George Anderson
[59]
Dr. Anderson holds a BSc in biology and chemistry from the University of Illinois and a PhD in medicinal chemistry from the University of Michigan. He was qualified as an expert in synthetic organic chemistry and process chemistry. He notably held the position of Group Leader and Principal Scientist at E.R. Squibb & Sons, and wrote the book Practical Process Research & Development.
[60]
At trial, Dr. Anderson opined about claim construction, anticipation, and obviousness. He signed an Expert Validity Report on August 28, 2019, a Responding Expert Report on November 9, 2019, and a Reply Expert Report on December 11, 2019 (exhibits 120, 121, and 122 respectively).
[61]
Dr. Anderson was a calm, compelling, and credible witness. He answered questions in a forthright manner whether the answers were favorable or unfavorable to Apotex. I give his opinion considerable weight.
(2)
Dr. Robert Michael Williams
[62]
Dr. Williams holds a BSc in chemistry from Syracuse University, and a PhD from the MIT. He was qualified as an organic and medicinal chemist. He worked all his life in academia and was an Emeritus Distinguished Professor at the Colorado State University.
[63]
Dr. Williams opined on the slate of issues before the Court. He signed an Expert Report on August 30, 2019, a Responding Expert Report on November 7, 2019 and a Reply Expert Report on December 11, 2019 (exhibits 126, 127, and 128 respectively).
[64]
Dr. Williams was an argumentative witness who appeared familiar with US law, but not so with Canadian law. The legal instructions for obviousness, anticipation, overbreadth, and inutility were not attached to his reports, and it appeared in fact, that he was not instructed on Canadian legal concepts. I am thus uncomfortable retaining his opinions that involved Canadian legal concepts, and will accordingly give them very little weight. As ruled at trial, parts of his report relying upon inadmissible discovery evidence, not otherwise produced at trial, are given less or no weight.
IV.
The 540 Patent
A.
Overview
[65]
The 540 Patent is titled “Modified Pictet-Spengler Reaction and Products Prepared Therefrom”
. It was filed (PCT) on July 14, 2003, published (PCT) on February 5, 2004, and issued on May 4, 2010. It claims priorities from US 60/400,386 (July 31, 2002), and US 60/460,161 (April 3, 2003), and will expire July 14, 2023.
[66]
LILLY ICOS, LLC, US is listed as the owner, and Mark W. Orme, Michael John Martinelli, Christopher William Doecke, Joseph Matthew Pawlak, and Erik Christopher Chelius are named as inventors.
[67]
The patent’s specification starts with the disclosure and ends with the claims.
B.
The disclosure
[68]
The disclosure is divided in four sections: (1) Field of the invention; (2) Background of the invention; (3) Summary of the invention; and (4) Detailed description of the preferred embodiments.
[69]
The Field of the invention section indicates that the invention relates to a modified PSR for introducing a second stereogenic center into a compound, and more particularly, to a modified PSR that provides a desired cis-or trans-diastereomer of a polycyclic compound having two stereogenic centers, in high yield and high purity.
[70]
The Background of the invention section starts by outlining the fact that compounds that exhibit biological activity typically contain at least one asymmetric carbon atom, ie at least one chiral center, and the importance of synthesizing the biologically active stereoisomers while minimizing or eliminating synthesis of the less active one. The benefits of stereochemical and optical purity and of stereoselective synthesis are outlined. It also outlines that many compounds contain two stereogenic centers, whereby the non hydrogen substituents of the asymmetric carbon atoms can be in a cis or a trans configuration, and that a particular problem in the synthesis of such biologically active compounds is the high yield and high purity preparation of a particular stereoisomer, which is the desired stereoisomer. A synthetic pathway must be provided to obtain the correct stereochemistry, high yield of the desired diastereomer in as few steps as possible, with a minimum of diastereomer separation and purification, which implies that there would still be diastereomer separation and purification steps in an ideal synthetic pathway.
[71]
The section goes on to refer to the US patent 5,859,006 (the 006 Patent) that discloses the synthesis of a compound I that has two asymmetric carbon atoms, each denoted by an asterisk, wherein the non-hydrogen substituents of the asymmetric carbon atoms are in the cis configuration. It details two pathways described in the 006 Patent wherein the key intermediate in the synthesis of compound I is compound II. It contains references, among other things, to a “step of separating”
(at page 2 line 18), a “diastereomer separation”
(at page 3 line 15). A “separation step”
(at page 4 line 21), and a difficult “product separation”
(at page 10 line 14).
[72]
Pathway A has few steps but the yield is poor, requires a separation step from the trans stereoisomer and utilizes trifluoroacetic acid (TFA). Pathway B provides a better yield but requires numerous synthetic steps. A key step in the synthesis of compound I is the preparation of compound II by the shorter synthetic pathway A by utilizing a PSR using D Tryptophan methyl ester and piperonal in dichloromethane acid at 4 degrees Celsius, and by obtaining the cis isomer by fractional crystallisation in 42% yield.
[73]
The Background of the invention section ends by indicating that it would be an important advance in the art to provide a modified PSR that substantially improves the diastereoselectivity of the reaction, so to ultimately overcome the disadvantages of the use of TFA, long reaction time and difficult product separation.
[74]
The Summary of the invention is said to be directed to a method of preparing a desired diastereomer, ie cis or trans, of a polycyclic compound having two asymmetric ring carbon atoms. It outlines that the method provides a good yield, shorter reaction times, avoids the use of TFA, uses a solvent in which the desired diastereomer is insoluble and the undesired one is soluble, and allows for equilibration that increases the yield of the desired diastereomer at the expense of the undesired one.
[75]
The Summary of the invention section also describes the preferred embodiments, outlining that the PSR is performed in a solvent in which the desired diastereomer is insoluble and the undesired one is soluble, utilizes an N-unsubstituted starting material, eg tryptophan, and eliminates the use of TFA. It specifies, twice, that the selection of the proper solvent is well within the skill of persons in the art (at page 13, lines 18 to 20; at page 14 lines 24 to 27) and mentions that isopropyl alcohol was found to solubilize the undesired diastereomer while the desired one precipitated, and that an equilibration results. It adds that the solubility difference allows for a fast and easy separation of the desired diastereomer from the undesired one, and that the equilibration allows for a more complete said separation (at page 14 lines 14 and 19). The section shows an example of the invention whereby each step includes filtration. I note particularly that the product of the SPR is isolated by crystallisation and filtration.
[76]
Finally, the section outlines the four steps of a detailed preparation example of Compound I. For step 2 of the process, which is the PSR, I note a seeding of Compound II in the disclosure of 0.05% to 0.25% based on the weight of D tryptophan methyl ester hydrochloride is preferred to induce crystallization, and it employs a cooling of the reaction mixture to 0 C before filtration, washing, and drying of the collected solid. In this step, the disclosure also presents variants of the solvents that could be used.
[77]
I note particularly the disclosure’s last paragraph, which reads: “Obviously, many modifications and variations of the invention as set forth above can be made without departing from the spirit and scope thereof, and therefore, only such limitations should be imposed as are indicated by the appended Claims”
.
C.
The claims
[78]
The 540 Patent’s specification ends with twelve claims, of which 8 are in issue, as Lilly are asserting Claims 1, 3–4, 7–10, and 12. Claims 1, 7 and 12 are independent and the remainder of the claims at issue are dependant on one of those independent claims.
[79]
The parties disagree on the interpretation of independent Claims 1, 7 and 12, and the disagreements echo in the dependant claims. As pointed out by Apotex, in a situation that appears somewhat odd, Lilly, the patentee, ask the Court to move away from the plain language of the claims while Apotex asks the Court to adhere to the plain language of the claims.
[80]
I will review the law of claim construction and determine how the PSA would read each claims and how they should be construed.
V.
Claim construction
A.
Relevant date for claim construction
[81]
The relevant date for claim construction of the 540 Patent is the date of publication, which is February 5, 2004.
B.
Law of claim construction
(1)
Introduction
[82]
The content of a patent specification is regulated by subsection 27(3) of the Patent Act. The first part is the disclosure, where the patentee must “fully describe the invention and its operation or use as contemplated by the inventor”
, “set out clearly the various steps in a process, […] in such full, clear, concise and exact terms as to enable any person skilled in the art, or science to which it pertains, or with which it is most closely connected, to make it”
, and in the case of a process, “explain the necessary sequence, if any, of the various steps, so as to distinguish the invention from other invention”
. As stated in Whirlpool (at para 42), the disclosure is the quid part of the bargain, provided to the inventor in exchange for the quo of a, now 20 year, monopoly on the exploitation of the invention.
[83]
The monopoly is enforceable, and it is thus important for the public to know what is prohibited, and where they may safely go, while the patent is still in existence. The public notice function is performed by the claims at the end of the specification which must “distinctly and in explicit terms define the subject-matter of the invention for which an exclusive privilege or property is claimed”
(Patent Act, subsection 27(4)).
[84]
An inventor is not obliged to claim a monopoly on everything new, ingenious and useful disclosed in the specification. The usual rule is that what is not claimed is considered disclaimed (Whirlpool at para 42; Monsanto Canada Inc v Schmeiser, 2004 SCC 34 at paras 122–123). If the inventor has misspoken, or otherwise created an unnecessary or troublesome limitation in the claims, it is a self-inflicted wound (Free World Trust at para 51).
[85]
Claims are not to be construed with extrinsic evidence with the exception of the common general knowledge that the skilled addressee already possesses. In December 2018, another exception was introduced, as section 53.1 was added to the Patent Act. It provides a limited exception to admit as evidence parts of communications between the patentee and the Patent Office during the prosecution of the patent, but only to rebut a representation by the patentee in an action (Canmar Foods Ltd v TA Foods Ltd, 2019 FC 1233 at para 68 [Canmar]).
(2)
One construction for all purposes
[86]
The first step in a patent suit is to construe the claims. This construction is antecedent to consideration of both validity and infringement issues and is the same for all purposes (Free World Trust at paras. 33-50; Whirlpool at paras 42-43; AstraZeneca Canada Inc v Apotex Inc. 2017 SCC 36 [AstraZeneca SCC] at para 31).
[87]
This was made clear in Whirlpool, where the appellants had argued that the two inquiries – validity and infringement – were distinct, and that if the principles of “purposive construction”
derived from Catnic Components Ltd v Hill & Smith Ltd, [1982] RPC 183 (UKHL) [Catnic] were to be adopted, they should properly be confined to infringement issues only. The principle of “purposive construction”
, they argued, had no role to play in the determination of validity. The SCC rejected this argument, as accepting it could result in a different claim construction for the purpose of validity than for the purpose of infringement, contrary to the fundamental rule of claim construction that the claims receive one and the same interpretation for all purposes (Whirlpool at para 49).
[88]
A claim cannot be construed with an eye on the allegedly infringing device in respect of infringement or with an eye to the prior art in respect of validity to avoid its effect (Dableh v Ontario Hydro, [1996] 3 FC 751 (FCA)).
[89]
Claim construction is a matter of law for the judge. The role of the expert is not to interpret the patent claims, but to put the trial judge in the position of being able to do so in a knowledgeable way; expert evidence regarding the construction of a patent claim is permissive, but not obligatory. (Whirlpool at para 61; Purdue Pharma v Canada (Attorney General) 2011 FCA 132 at para 16). Claims should be construed by the PSA, as of the date of the publication, based on his or her common general knowledge.
[90]
Finally, the canons of the law of claim construction have been set by the SCC in Consolboard Inc v MacMillan Bloedel (Saskatchewan) Ltd, [1981] 1 SCR 504 (SCC) at 520-525, Free World Trust, and Whirlpool. Although these decisions pertained to patents covered by a previous version of the Patent Act, they do apply (see for example Cobalt Pharmaceuticals Company v Bayer Inc, 2015 FCA 116 [Cobalt]).
(3)
Purposive construction: essential and non-essential elements
[91]
In both Whirlpool and Free World Trust, the SCC retained the purposive construction approach. By doing so, the SCC rejected the so-called “two-step”
approach to patent construction, whereby courts first considered whether on a literal construction the allegedly infringing device embodied the patented invention and, if not, whether that device embodied the “pith and marrow”
or “substance”
of the invention (Canamould Extrusions ltd v Driangle inc 2004 FCA 63 para 20 [Canamould Extrusions]).
[92]
The single-step, or purposive, approach was preferred because “the greater the level of discretion left to courts to peer below the language of the claims in search for 'the spirit of the invention', the less the claims can perform their public notice function, and the greater the resulting level of unwelcome uncertainty and unpredictability”
(Free World Trust at para 50). That approach, as enunciated by Lord Diplock in Catnic, calls for a “purposive construction”
of a patent. It was applied by the FCA in Eli Lilly & Co v O'Hara Manufacturing Ltd (1989), 26 CPR (3d) 1 (FCA).
[93]
In Whirlpool, the SCC stated that purposive construction properly directs itself to the words of the claims interpreted knowledgeably and in the context of the specification as a whole, and advances the objective of an interpretation of the patent claims that is reasonable and fair to both patentee and public. The SCC specified that the key to purposive construction is the identification, by the court, with the assistance of the skilled reader, of the particular words or phrases in the claims that describe what the inventor considered to be “essential”
elements of his invention (at paras 49, 45).
[94]
In fact, claim elements are presumed to be essential, and a party alleging otherwise bears the onus of establishing non-essentiality (Mediatube Corp v Bell Canada, 2017 FC 6 at para 33 [Mediatube]).
[95]
In Free World Trust, the SCC provided additional guidance on how to determine essential and non-essential elements of the claims. I note that the SCC’s guidance in this regard was provided mainly while it was addressing the infringement issues, and only after it had, at paras 20-23, construed the claims. Understandably, the SCC thus provides guidance both on how to distinguish the essential from the non-essential elements as it pertains to claim construction, and on how this determination affects the infringement analysis. Those two aspects appear intertwined, and, at para 55, the SCC confirms that the elements of the invention are identified as either essential elements (where substitution of another element or omission takes the device outside the monopoly), or non-essential elements (where substitution or omission is not necessarily fatal to an allegation of infringement). Hence, if an element is construed as being essential, its substitution will take the defendant outside the realm of the monopoly, and there will be no infringement.
[96]
Since the Court must construe the claim without regard to the infringement or validity issues, I will, thus for now, identify the elements of the Free World Trust decision that guide claim construction. Importantly, the claims language will, on a purposive construction, show that some elements of the claimed invention are essential while others are non-essential. As per paragraph 31 of Free World Trust, the identification of elements as essential or non-essential is to be made:
i.
on the basis of the common knowledge of the worker skilled in the art to which the patent relates;
ii.
as of the date the patent is published;
iii.
having regard to whether or not it was obvious to the skilled reader at the time the patent was published that a variant of a particular element would not make a difference to the way in which the invention works; or
iv.
according to the intent of the inventor, expressed or inferred from the claims, that a particular element is essential irrespective of its practical effect;
v.
without, however, resort to extrinsic evidence of the inventor's intention.
[97]
The SCC examined each of those five points at paras 51 to 67 of the decision.
[98]
As part of the exam of components iii and iv, the SCC confirmed that for an element to be considered non-essential, it must be shown either (i) that on a purposive construction of the words of the claim, it was clearly not intended to be essential, or (ii) that at the date of publication of the patent, the skilled addressees would have appreciated that a particular element could be substituted without affecting the working of the invention, i.e., had the skilled worker at that time been told of both the element, specified in the claim, and the variant and “asked whether the variant would obviously work in the same way”
, the answer would be yes (Free World Trust at para 55).
[99]
The SCC referred to the decision of Improver Corp v Remington Consumer Products Ltd, [1990] FSR 181 (Pat Ct), and cited Justice Hoffmann, himself citing Catnic, and his three questions, now referred to as the Improver questions:
i.
Does the variant have a material effect upon the way the invention works? If yes, the variant is outside the claim. If no: –
ii.
Would this (i.e.: that the variant had no material effect) have been obvious at the date of publication of the patent to a reader skilled in the art? If no, the variant is outside the claim. If yes: –
iii.
Would the reader skilled in the art nevertheless have understood from the language of the claim that the patentee intended that strict compliance with the primary meaning was an essential requirement of the invention? If yes, the variant is outside the claim.
[100]
It does not appear, as Lilly presented it in its closing oral arguments, that Justice Hoffmann’s three questions were “to sort out a test for the Court in order to make that determination of would something work substantially the same way and give substantially the same result”
. It appears these questions have been formulated first and foremost to assist the Court identify the essential and the non-essential elements of the claims. In Canamould Extrusions, the FCA noted the perspective Justice Hoffmann added at page 190 of his Improver decision where he indicated, essentially, that the first two questions do not primarily involve construction, they provide factual background, their answers are not conclusive, and that it is the third question, related to the patentee’s intention, which raises the question of construction.
[101]
Justice Scott in Hollick Solar Systems Ltd v Matrix Energy Inc, 2011 FC 1213 at paras 54-82 and Justice Locke in Mediatube at paras 33-34, 52, both applied Improver as part of their claim construction in order to identify the essential and non-essential elements.
[102]
In regards to the intention of the inventor, the SCC indicated that “The courts recognize the pitfalls of language and will do what they can to give the inventor ‘protection for that which he has actually in good faith invented’ (Western Electric, supra, at p. 574), but there are limits”
. Citing the FCA, the SCC added that a court must interpret the claim and cannot redraft them. When an inventor stated in the claim that he considered a requirement as essential to his invention, a court cannot decide otherwise for the sole reason that he was mistaken (Free World Trust at paras 58–59).
[103]
Justice Hoffmann stressed that point in Improver, when he concluded that “Even a purposive construction of the language of the patent may lead to the conclusion that although the variant made no material difference and this would have been obvious at the time, the patentee for some reason was confining his claim to the primary meaning and excluding the variant. If this were not the case, there would be no point in asking the third question at all”
(at 190). It is worth noting that he so concluded even if the patent under review contained an “equivalents clause”
.
(4)
Purposive construction: the patentee’s words
[104]
Words chosen by the inventor must be read in the sense the inventor is presumed to have intended and in a way that is sympathetic to accomplishment of the inventor’s purpose expressed or implicit in the text of the claims. Again, claims are to be read in an informed and purposive way with a mind willing to understand, viewed through the eyes of the person skilled in the art as of the date of publication having regard to the common general knowledge.
[105]
Courts have traditionally protected a patentee from the effects of excessive literalism. It is unsafe in many instances to conclude that a term is plain and unambiguous without a careful review of the specification (Whirlpool at para 52). When applying a purposive construction of claims, the court must look at the specification of the patent for the meaning of a word before looking in dictionaries. A patentee is entitled to be his, her or its own lexicographer (Kramer v Lawn Furniture Inc (1974), 13 CPR (2d) 231 at 237 (FCTD); Pfizer Canada v Canada (Minister of Health), 2005 FC 1725 at para 19; Minerals Separation North American Corp v Noranda Mines Ltd (1952), 15 CPR (1st) 133 at 144–145 (Priv Coun)).
[106]
The patent specification “is not addressed to grammarians, etymologists or to the public generally, but to skilled individuals sufficiently versed in the art to which the patent relates to enable them, on a technical level, to appreciate the nature and description of the invention”
(H. G. Fox, The Canadian Law and Practice Relating to Letters Patent for Inventions, 4th ed, (Toronto: Carswell 1969) at 185). As per the words of Dr. Fox, the Court must place itself “in the position of some person acquainted with the surrounding circumstances as to the state of the art, and the manufacture at the time, and making itself acquainted with the technical meaning in that art or manufacture that any particular word or words may have”
(Whirlpool at para 53). The FCA has recently cited this passage from Whirlpool in AFD Petroleum Ltd v Frac Shack Inc, 2018 FCA 140 at para 60.
[107]
However, “the purposive approach is not an invitation to the Court to ignore the ordinary rules of grammar and syntax”
(ABB Technology AG v Hyundai Heavy Industries Co, Ltd, 2015 FCA 181 at para 45, aff’g 2013 FC 947).
[108]
While Free World Trust adopts the purposive construction approach, it also confirms that the Patent Act, as it then read, promotes adherence to the language of the claims.
[109]
In a more recent decision, the FCA in Tearlab v I-MED Pharma Inc, 2019 FCA 179 at para 47 [Tearlab FCA] approved the trial judge’s construction and his adherence to the words of the claims. The trial judge refused to add limitations that were not expressly included and focused on the claims without redrafting them. The FCA also reiterated that, although consideration can be given to the patent specifications to understand what was meant by the words in the claims, one must be wary not to use these so as “to enlarge or contract the scope of the claim as written and understood”
(at paras 32–34).
[110]
In Hospira Healthcare Corporation v Kennedy Trust for Rheumatology Research, 2020 FCA 30, [Hospira FCA] the FCA also saw no error in the Federal Court judge’s decision to interpret the words of the claims to have their plain meaning and to look at the disclosure for assistance in their construction given the arguments raised by the appellants. In that particular case, the appellants, which were the ones sued for infringement, sought to limit ambits of the claims although the claims contained no explicit limitation, and the disclosure confirmed that there were no such limitations.
(5)
Claim differentiation
[111]
The concept of claim differentiation presumes that patent claims are drafted as not to be redundant and that each different claims have different scopes (Donald Cameron, Canadian Patent Law Benchbook, 3rd Ed (Toronto: Thomson Reuters, 2019); Halford v Seed Hawk Inc, 2004 FC 88, aff’d 2006 FCA 275). The rebuttable presumption that claims are not redundant was first applied between a claim and its dependant claims (Apotex Inc v Lundbeck Canada Inc, 2010 FCA 320 at para 110; Bridgeview Manufacturing Inc v 931409 Alberta Ltd (Central Alberta Hay Centre), 2010 FCA 188; ViiV Healthcare Company v Gilead Sciences Canada, Inc, 2020 FC 486 at para 56). It is now also applied in claim differentiation between independent claims (Camso Inc v Soucy International Inc, 2019 FC 255 at paras 103, 186–190).
[112]
Claim differentiation is useful to determine whether a claim element is essential. Hence, where one claim differs from another in only a single feature, it is difficult to argue that the different feature has not been made essential to the claim (Whirlpool at para 79). It would be peculiar that the inventor intended for two claims to be redundant.
[113]
If an essential feature of a claim is defined in a specific way and a different more expansive term is also introduced that can include the specific term, one would not generally interpret the two terms as denoting the same thing. The usual purpose of using different words is to distinguish one feature from another and not to express synonyms (ABB Technology AG v Hyundai Heavy Industries Co Ltd, 2013 FC 947 at para 29, aff’d 2015 FCA 181).
C.
Person skilled in the art
[114]
A patent is to be construed through the eyes of a person of ordinary skills in the art (PSA), who is not an inventor (Beloit Canada Ltd v Valmet OY (1986), 8 CPR (3d) 289 (FCA) at 294 [Beloit]). The parties do not have any substantial disagreement as to who the PSA is in this case. For Lilly, the PSA is an organic chemist with experience in synthetic organic chemistry (Lilly Closing Memorandum at page 6). For Apotex, the PSA is a chemist or chemical engineer responsible for the synthesis and manufacturing of drug substances (Apotex Closing Memorandum at page 12). The parties essentially agree that the 540 Patent is directed to a chemist or chemical engineer in the pharmaceutical industry responsible for the synthesis and manufacturing of drug substances.
[115]
Lilly argue that the parties’ disagreement lies rather in the grammarian approach Apotex seeks to add to the purposive construction, referring namely to gerunds or action words. They insist that the PSA is not a grammarian or a general member of the public. I agree with Apotex, that although not a grammarian, the PSA, being a chemist of chemical engineer, would still have completed high school grammar.
D.
Prior art
[116]
Prior art is “the collection of learning in the field of the patent at issue”
and “comprises any publically available teaching, however obscure or not generally accepted”
(Mylan Pharmaceuticals ULC v Eli Lilly Canada Inc, 2016 FCA 119 [Mylan Pharmaceuticals FCA] at para 23).
[117]
Ciba Specialty Chemicals Water Treatments Limited’s v SNF Inc, 2017 FCA 225 at para 56 [Ciba FCA] confirms that state-of-the-art is simply another term for prior art, and I may thus use both terms in these reasons. The FCA recently held that no public piece of art should be excluded from the prior art solely because it could not be located following a reasonable diligent search (Hospira FCA at para 86).
[118]
In this case, Apotex has identified the 594 Application as the piece of prior art for the allegations of anticipation, and the 377 Patent (or its US equivalent, the 006 Patent) and the 594 Application as pieces of prior art for the allegations of obviousness.
[119]
Lilly do not dispute Apotex’ pieces of prior art, but they seek to bring in the 2,411,008 Application (008 Application) as prior art for the purpose of the obviousness analysis. They argue it teaches away from the invention, by disclosing a poorly yielding PSR of a tryptophan derivative with isopropyl alcohol as solvent. While it is the responsibility of the person alleging obviousness to point to those specific elements of the prior art, this does not give them “free rein to define the state of the prior art”
(Frac Shack Inc v AFD Petroleum Ltd, 2018 FC 1047 at para 54, rev’d in part on other grounds 2018 FCA 140). Consequently, Lilly is entitled to assert the 008 Application as a piece of prior art.
(1)
The 594 Application
[120]
The 594 Application, filed by Lilly ICOS LLC, US, is titled “Chemical Compounds”
. The inventors listed on the Application are Mark W. Orme, Jason Scott Sawyer, Lisa M. Schultze, Alain Claude-Marie Daugan, and Francoise Gellibert. The Application was filed on May 15, 2001, claiming priority from US Patent 60/213,647 filed on June 23, 2000. In Canada, it was first published in January 3, 2002. The 594 Patent was ultimately issued on March 17, 2009, after the relevant priority date of the 540 Patent, so only the Application published beforehand is prior art.
[121]
The 594 Application describes the preparation of a number of compounds for the inhibition of PDE5, notably of an analogue of tadalafil.
[122]
Apotex invokes the preparation of Intermediate 1 of Example 2 at page 34 of this piece of prior art to attack, on the basis on anticipation, the validity of Claims 1, 3-4 of the 540 Patent and, on the basis of obviousness, the validity of all of the asserted Claims. A PSR reaction is disclosed. Essentially, the initially suspended D-tryptophan ester hydrochloride is reacted with piperonal in acetic acid and water in a 50:1 ratio. The resulting suspension is cooled, and anti-solvents are added, before isolation of the desired cis. Apotex asserts that the resulting suspension indicates that the desired cis crystallized, whereas Lilly deny that the 594 Application discloses the suspension to be the desired cis and argue there is no disclosure on the thickness of the suspension.
(2)
The 377 Patent
[123]
The 377 Patent is titled “Tetracyclic Derivatives, Process Of Preparation and Use”
. The name inventor of the Patent is Dr. Alain Claude-Marie Daugan. The application was filed on January 19, 1995, claiming priority from UK Patent 9401090.7 filed on January 21, 1994. In Canada, it was published on July 27, 1995, and was issued in May 28, 2002. In the US, it is labelled as the 006 Patent.
[124]
Informally, the parties refer to the Canadian 377 Patent and to the US 006 Patent as the Daugan Patent. Apotex invokes it to attack the validity of all the asserted Claims of the 540 Patent on the basis of obviousness. The 377 Patent describes a process, in intermediates 54 and 55 at page 23 and in intermediates 67 and 68 at page 25, where D-tryptophan methyl ester is reacted with piperonal in dichromethane in the presence of trifluoroacetic acid. It also describes processes to convert the undesired trans-diastereomer, or a mixture of cis and trans, into cis or a mixture in the presence of an acid in specific conditions in intermediate 69 at page 26.
(3)
The 008 Application
[125]
The 008 Application, filed by Lilly ICOS LLC, US, is titled “Derivatives of 2,3,6,7,12,12A-Hexahydropyrazino [1’,2’:1,6] Pyrido [3,4-B] Indole-1,4-Dione”
. The inventors listed on the Application are Mark W. Orme, Jason Scott Sawyer and Alain Claude-Marie Daugan. The Application was filed on May 15, 2001, claiming priority from US Patent 60/210,137 filed on June 7, 2000.
[126]
In Canada, it was first published in December 13, 2001. The 008 Application describes the preparation of a number of compounds for the inhibition of PDE5, notably of an analogue of tadalafil, but with a hydroxyl group on the benzene ring. An initial PSR reaction is required. A 5 -hydroxy-DL-tryptophan is reacted with piperonal in dichloromethane in the presence of trifluoroacetic acid as catalyst.
[127]
Lilly raise this piece of prior art as part of the obviousness analysis to argue that it teaches away from the 540 Patent, as the yield of the PSR in isopropyl alcohol as a solvent is poor and the reaction is not stereoselective.
E.
Common general knowledge
[128]
Common general knowledge does not amount to all information in the public domain. Rather, common general knowledge is the knowledge generally known at the relevant time by the person skilled in the field of art or science to which the patent relates (Bell Helicopter Textron Canada Limitée v Eurocopter, société par actions simplifiée, 2013 FCA 219 at paras 63–65 [Bell Helicopter Textron]).
[129]
The assessment of common general knowledge is governed by the principles found in Eli Lilly & Co v Apotex Inc, 2009 FC 991 at para 97 [Eli Lilly 2009], aff'd 2010 FCA 240, citing General Tire & Rubber Co v Firestone Tyre & Rubber Co, [1972] RPC 457 (UKHL) at 482-483:
1) Common general knowledge is distinct from what in patent law is regarded as public knowledge. Public knowledge is theoretical and includes each and every patent specification published, however unlikely to be looked at and in whatever language it is written. Common general knowledge, in contrast, is derived from a common sense approach to the question of what would be known, in fact, to an appropriately skilled person that could be found in real life, who is good at his or her job.
2) Individual patent specifications and their contents do not normally form part of the relevant common general knowledge, although there may be specifications which are so well known that they do form part of the common general knowledge, particularly in certain industries.
3) Common general knowledge does not necessarily include scientific papers, no matter how wide the circulation of the relevant journal or how widely read the paper. A disclosure in a scientific paper only becomes common general knowledge when it is generally known and accepted without question by the bulk of those engaged in the particular art.
4) Common general knowledge does not include what has only been written about and never, in fact, been used in a particular art.
[130]
In other words, as stated in Mylan Pharmaceuticals FCA “common general knowledge […] is the knowledge generally known by persons skilled in the relevant art [skilled persons] at the relevant time”
. Unlike the prior art, which is a broad category encompassing all previously disclosed information in the field, a piece of information only migrates into the common general knowledge if a skilled person would become aware of it and accept it as “a good basis for further action”
.
[131]
A PSA’s common general knowledge cannot be assumed; rather, it must be proven with fact evidence on a balance of probabilities (Eli Lilly 2009 at para 100).
[132]
The relevant date for assessing common general knowledge for the purpose of claim construction is the publication date, February 5, 2004. However, the relevant date for assessing common general knowledge for the purpose of the obviousness and anticipation analysis is the claim date, which is July 31, 2002.
[133]
Dr. Laird, Lilly’s expert, outlined the common general knowledge as follows, except for the last point:
·
Initially, a medicinal chemistry process with poor yield may be acceptable, and to enhance the yield, there are mainly three solutions: (1) use a different and better synthetic route to the API; (2) use a different and better route to a key intermediate of the API; (3) enhance the medicinal chemistry synthetic route by changing the reagent, adding certain reactants, changing the rate of addition, reordering the steps, or changing the reaction conditions.
·
The discoverer of a molecule may also seek to claim the process to make an analogue in in the hopes that the analogue may bring better activity and more acceptable properties for a drug.
·
The purpose is to obtain the API in acceptable yield and purity in a way that is cost effective, robust, scalable, easy to perform by non-chemists, and minimally polluting.
·
Stereochemistry is important and properties of stereoisomers are often different. A PSR would result in a mixture of diastereomers.
·
Commenting as well on the conversion of the tetrahydro-β-carboline to tadalafil, Dr. Laird pointed out the 377 Patent outlines the process, and that the process in the 594 Application is used to make an analogue. He also commented that the 377 Patent disclosed a process in which the cis-diastereomer was more soluble.
·
Crystallisation on a large scale is a tricky operation, and the crystallisation of mixtures is difficult to achieve in high yield, and pure diastereomers are only obtained after multiple crystallisations.
·
In protic solvents, there is little control of the stereochemistry in a PSR, and Dr. Laird gave the 008 Application as an example of a PSR in isopropyl alcohol, which is a protic solvent with poor stereoselectivity. He therefore opined that the PSA would understand that the PSR can be carried out under either aqueous acidic media or aprotic conditions. J.M. Cook reviewed the literature and noted that a PSR can be carried out in aprotic media. P.D. Bailey also studied tryptophan methyl ester with aldehyde and noted that certain reactions in aprotic media can give a 80:20 cis-trans ratio, which is good in academic settings, but may not be good commercially. Bailey also noted that higher temperature favored the formation of trans, using L-tryptophan. Referring to the 377 Patent, Dr. Laird postulated that the trans seems less soluble than the cis. He also noted that the 377 Patent indicated that the trans would convert into a mixture of cis and trans; this is despite the fact that Bailey observed the cis turn to trans on N-benzyl derivatives of tryptophan, which Dr. Laird admitted as being chemically different from tryptophan.
[134]
Dr. Anderson, Apotex’s expert did not particularly opine on what form part of the common general knowledge, but answered questions addressing what a skilled process chemist would do in a given situation as of July 31, 2002:
·
When adapting a synthetic process for use in the manufacturing of an API on a commercial scale, a skilled chemist would seek to optimize the safety, reliability, cost-effectiveness, efficiency, yield, and environmental impact of the process and the purity of the product;
·
To adapt the process to a commercial scale, the chemist would typically modify, vary or substitute the synthetic process, reagents or solvents, the temperatures at which the reactions are conducted as well as the manner in which the product is isolated and the number of operations;
·
If the process outlined in pages 25–26 of the 377 Patent were given to the skilled process chemist for adaptation, skilled process chemist would: 1) strongly prefer to employ another acid instead of trifluoroacetic acid; 2) minimize the number of extractions and the volume of solvent required; 3) reduce the number or eliminate altogether operations involving the transfer of solvent; 4) crystallize the cis‐diastereomer first; and 5) avoid the use of isopropyl ether;
·
If the process outlined in page 34 of the 594 Application were given to the skilled chemist for adaptation, the skilled process chemist would endeavor to increase the yield of the desired product and reduce reaction times to increase the space-time yield, by notably refining the solvent and reagent choice and reduce, where possible, the steps used to isolate the product by crystallization.
F.
Claims needing construction
(1)
Introduction
[135]
As mentioned earlier, the independent claims needing constructions are Claims 1, 7 and 12. More specifically, the construction will be focused on “where the shoes pinches”
(Cobalt at para 83).
(2)
Construction of Claims 1, 3-4
(a)
The claim in dispute
[136]
Claim 1 is an independent claim, and Claims 3 and 4, also asserted, are among its dependant claims. Claim 1 is reproduced hereafter:
A method of preparing a desired cis-diastereomer of a
tetrahydro-β-carbolinehaving a formula
comprising the steps of:
a) providing a tryptophan esterified using an alcohol having a formula R2 OH wherein R2 is aselected from methyl, ethyl, isopropyl, n-propyl, n-butyl, sec-butyl, t-butyl, and mixtures thereof;
b) reacting, the tryptophan ester of step (a) with an aldehyde having a formula R1CHO wherein R1 is piperonyl, to provide the desired disatereomer and an undesired diastereomer wherein the reaction is performed in a solvent in which the desired diastereomer is insoluble at reflux temperature or lower and the undesired diastereomer is soluble at reflux temperature or lower; and
c) separating the insoluble desired diastereomer from the soluble undesired diastereomer.
[137]
The parties disagree on the construction of Claim 1. I have emphasized the words or section that are in play, ie were the “shoe pinches”
.
(b)
Claim 1 first paragraph: “a desired cis-diastereomer”
versus “the desired cis-diastereomer”
[138]
The first contentious issue pertain to the patentee’s use of the article “a”
rather than the article “the”
in the first paragraph. Despite their disagreement, the parties recognise that this use has no effect on either invalidity or infringement. Apotex confirmed that “while nothing may ultimately turn on the proper construction of the phrase ‘a desired cis-diastereomer of a tetrahydro-β-carboline’ in this case, the Plaintiffs’ argument in this respect is illustrative of the incorrect approach to construction the Plaintiffs and Dr. Laird have taken throughout”
(Apotex Closing Memorandum at para 19). Lilly also concede that it is not important to focus on the dispute on the definite or indefinite articles “a”
versus “the”
, and the meaning of desired cis-diastereomer in Claim 1. Claim 4 is limited to D-tryptophan and as such only the R,R version is made, and Lilly argue that Apotex’s cross-examination of Dr. Laird on definite and indefinite articles is totally irrelevant. Despite these comments made in their respective closing submissions, both parties stood on their grounds and made full submissions to the Court on the matter.
[139]
Lilly suggest that Claim 1 refers to the method of making the R,R cis-diastereomer with the D-tryptophan. They contend that the patentee’s use of an indefinite article was dictated by the rules contained in the Manual of Patent Office Practice, and that the use of a definite article in these circumstances would not have been permitted because the noun “cis desired diastereomer”
had not been properly introduced. Lilly qualify Apotex’s suggestion that it could be the R,R or S,S variants as an example of the improper grammarian approach Apotex adopts.
[140]
Apotex argues that the use of the indefinite article “a”
, rather than the definite article “the”
, indicates the patentee referred, in Claim 1, to a method of preparing both cis-diastereomers, ie the R,R and the S,S configurations, and did not limit Claim 1 to the R,R cis-diastereomer configuration. Apotex relies on (1) the usual meaning of an indefinite article whereby it refers to the non-specific noun that follows; (2) the fact that Claim 1b does not specify the type of tryptophan that must be used, which serves to confirm that the cis-diastereomer could be either the R,R or the S,S variant since different types of tryptophan would result in different configurations, showing no precise stereochemistry; and (3) the fact that Claim 4, which depends on Claim 1, narrows the tryptophan in step b to the D-tryptophan, which would lead to the R,R configuration, which, applying the principle of claim differentiation, directs us to conclude that Claim I is not limited to the R,R configuration.
[141]
The Court must strive for a construction that is not redundant (Camso Inc v Soucy International Inc, 2019 FC 255 at paras 103, 186–190).
[142]
Even if I adopt Lilly’s position in regards to the proper use of an indefinite article, as directed by the Patent Office, thus accepting that the desired cis-diastereomer was not properly introduced in the disclosure, I cannot rally to their overall position that Claim 1 only refers to the R,R cis configuration.
[143]
My decision does not rely on grammar, but on the fact that (1) Claim 1b does not specify the type of tryptophan that must be used, which serves to confirm that the cis-diastereomer could be either the R,R or the S,S variant since different types of tryptophan would result in different configurations, showing no precise stereochemistry; and (2) Claim 4 , which is dependant on Claim 1, narrows the tryptophan in step b to the D-tryptophan, which leads to the R,R configuration, which, applying the principle of claim differentiation, directs the Court to strive for a construction of Claim 1 that is not redundant.
[144]
This leads me to conclude that Claim 1 is directed to a desired cis-diastereomer, without specifically referring to the R,R configuration. This precise R,R configuration is achieved by the use of the D-tryptophan at Claim 4.
(c)
Claim 1 step b: solvents
[145]
Lilly argue that Claim 1b provides a functional limitation to the solvents covered by the claim, and that this functional limitation defines the essential element.
[146]
The solvent must be such that the desired cis-diastereomer is substantially insoluble, and the undesired trans-diastereomer is soluble, allowing the interconversion of trans diastereomers into cis, driving the process to precipitate more of the desired cis-diastereomer. Lilly submit that the patentee makes it very clear that this is an overarching requirement for success of the invention, that the patentee does not have to claim specifically all solvents in its specification and not all of the solvents disclosed in the patent may meet the functional limitation set out in Claim 1b. For Lilly, the selection of the solvent for the present modified PSR is within the skills of the PSA and taught by the 540 Patent. Lilly point particular to page 14 of the disclosure at lines 24–27.
[147]
Apotex does not respond directly to Lilly’s claim construction on the matter. However, it asks the Court to adhere to the words of the claim, and stresses that nothing in the claim describes a CIAT, equilibration, high yields, high purity, and rapid reaction.
[148]
I agree with Lilly that Claim 1b provides a functional limitation to the solvents covered, in that, as per the text of the claim, the solvent must be such that the desired cis-diastereomer is insoluble at reflux temperature or lower, and the undesired trans-diastereomer is soluble at reflux temperature or lower.
[149]
I note that neither Lilly nor its expert assert the results, or benefits of the PSR reaction as essential elements of Claim 1. There is no request from Lilly to construe the cis trans equilibration CIAT, high yield, high purity, faster processing and fewer steps as part of Claim 1. They are not construed in the claim.
(d)
Claim 1 step c: phase separation vs separation from the mixture
[150]
Lilly argue that Claim 1c simply requires that there be the separation of the cis-diastereomer from the trans-diastereomer in the mixture through precipitation or crystallisation, and argue that Claim 1c does not refer to a step of physical separation (filtration or isolation) of the cis-diastereomer from the mixture. Lilly contend that the clear language of the claim and the patent support their position. They direct the Court to page 14, lines 6 to 23 of the disclosure where the “separation” of the desired diastereomer from the undesired diastereomer is mentioned twice within the same paragraph. They add that these are the only occasions in the specification where the inventors use a form of the word “separate”
to describe the invention and not the prior art. For Lilly, this construction only requires one diastereomer to separate from the other, as a result of the solubility difference of the reaction products in Claim 1b.
[151]
Lilly acknowledge that the words separating or separation, used by a chemist, can mean a number of things, and they recognise that experts have discussed, at the hearing, using separation as a term to designate phase separation, or separation from the mixture by chromatography or filtration for example. To a chemist, all of those things can mean separation, but as far as the patent is concerned, Lilly assert that the patentee indicated at page 14 that separation meant the separating of one compound from the other.
[152]
Lilly also stress that “separation”
in the meaning of “filtration”
is not explicitly outlined in the claims, as it forms part of the common general knowledge, which explains why it is not mentioned in Claim 1a and why other claims in the patent do not include this filtration step.
[153]
Lilly further argue that Apotex’s position is again an improper grammarian one, resorting to gerunds and to actions words to suggest human operations, which is unsupported by the language of the patent as a whole, and that Apotex seeks to read in the step to avoid infringement.
[154]
Lilly submit that a purposive construction leads to construing Claim 1c as a phase separation and that the essential elements of Claim 1 are thus as Dr. Laird stated them to be in his report.
[155]
Alternatively, if Claim 1c is to be construed as an isolation or filtration step as suggested by Apotex, Lilly submit that it is a non-essential element, the essential claimed invention being that the desired diastereomer crystallizes in the reaction. For Lilly, nothing requires the “desired cis-diastereomer”
to be isolated or be free of other components of the reaction mixture; it simply needs to be prepared, and a filtration step can be omitted without having a material effect on either the structure or the operation of the invention (Free world Trust at para 20). No witness has supported this position, but Lilly argue they are allowed to put that argument to the Court and the Court is allowed to construe the claims without reference to any expert testimony at all. I agree that Lilly can submit this argument.
[156]
Apotex, on the other hand, argues that Claim 1c is a step of separating the crystallized diastereomer from the mixture, by filtration for example, and that it is not a phase separation within the mixture. Apotex points to the structure of Claim 1 and the meaning of the words, including the use of “separating”
as opposed to “separation”
. For Apotex, the objective of Claim 1 is precisely to prepare a desired cis-diastereomer and not a mixture of desired and undesired diastereomer.
[157]
Apotex adds this is confirmed by the fact that the desired cis-diastereomer must be available as the starting material to the process of Claim 7, and that the desired cis-diastereomer must be the cis-diastereomer in the R,R configuration; otherwise, tadalafil cannot be made going into Claim 7, as confirmed by Dr. Laird (transcript of January 17, 2020 at page 23). Clearly, the desired cis-diastereomer must be filtrated or isolated, ie separated from the mixture at the end of the process of Claim 1. Apotex consequently submits this separating step is a usual step performed by a chemist in preparing a compound.
[158]
Apotex submits that the clear language, structure, punctuation and words indicate Claim 1c to be a step, as are Claims 1a and 1b, and that nothing justifies conflating step c into step b, particularly since no other steps in the claims are so conflated together. “Separating”
, a gerund denoting an action, and “separation”
are not the same words. Furthermore, Apotex submits that Lilly’s construction is redundant since at the end of step b, the two phases are already reacted, one diastereomer is already soluble and the other, insoluble, and the two distinct phases have already formed.
[159]
As parties confirmed that, for a skilled chemist, a phase separation is not the only form of separation, which would also include crystallization, Apotex points that “isolated”
, and “crystallization and filtration”
, as used in pages 17 and 21 of the disclosure, also designate the physical act of “separating.”
[160]
Apotex also takes the position that all three steps are essential elements because of the wording of the claim. Additionally, the product of Claim 1 is brought to Claim 7, and crystallized products in a mixture are not a starting point for the next steps in Claim 7.
[161]
I find that the language of Claim 1c refers to a step of separating the cis-diastereomer from the mixture, and not to a passive phase separation, which is already contemplated at Claim 1b. My finding is not displaced by the two mentions of a “separation”
at page 14 of the disclosure, referring to a fast and easy, and a more complete separation of the desired diastereomer from the undesired diastereomer. Lilly ignore all the references to separation and to steps of separation and separating mentioned elsewhere in the disclosure, as well as references to filtration and isolation steps in the description of the invention. In fact, the disclosure contains a number of mentions of step of separating or of separation that are not limited to a phase separation, namely at page 3 line 15–16, at page 4 line 21 (despite the absence of phase separation in the process being discussed) and at page 10 line 14. Lilly have not demonstrated that the patentee used a particular lexicography and intended the word “separation”
to mean only phase separation, nor that the separating step of Claim 1c is understood to be a phase separation. To the contrary, the patentee has included steps of filtration and isolation in the disclosure’s description of the PSR reaction leading to the making of compound II as drawn in page 7 of the 540 Patent at page 17 lines 6, 10–12.
[162]
The language of Claim 1 refers to steps, the last being one of separating. Basic grammar is not reserved to grammarians or linguist, and it leads me to conclude it is the action of separating the cis-diastereomer from the mixture; and nothing in the disclosure displaces this finding. My conclusion is based on the clear structure and language suggesting that Claim 1c is a separating step, meaning a human intervention step of filtering the mixture to isolate the desired diastereomer. This is confirmed by the fact that the reaction already reaches completion at the end of Claim 1b, that Claim 7 starts with the isolated compound, and that the other claims all have “steps”
that are action driven.
[163]
Lilly argue that Claim 1c, as construed is non-essential. Lilly bear the burden to show a claim is non essential (ViiV Healthcare Company v Gilead Sciences Canada, Inc, 2020 FC 486 at para 22; Mediatube at para 33), and they have not convinced me so, particularly given the fact that I construe the purification in Claim 7d as an essential step, as detailed below. I acknowledge that Claim 12, which is a narrower claim overlapping with other claims of the 540 Patent, has no purification step, but given the fact that Claim 7 has an essential purification step, Lilly have not convinced me that the patentee intended for Claim 1c to be non-essential.
(e)
Essential elements of Claims 1, 3-4
[164]
I thus construe Claim 1 as follows:
A method of preparing a desired cis-diastereomer (R,R or S,S) of a
tetrahydro-β-carbolinehaving a formula:
comprising the steps of (each steps being essential):
a) providing a tryptophan esterified using an alcohol having a formula R2 OH wherein R2 is selected from methyl, ethyl, isopropyl, n-propyl, n-butyl, sec-butyl, t-butyl, and mixtures thereof;
b) reacting, the tryptophan ester of step (a) with an aldehyde having a formula R1CHO wherein R1 is piperonyl, to provide the desired disatereomer and an undesired diastereomer wherein the reaction is performed in a solvent in which the desired diastereomer is insoluble at reflux temperature or lower and the undesired diastereomer is soluble at reflux temperature or lower (provides a functional limitation to solvent choice); and
c) separating the insoluble desired diastereomer from the soluble undesired diastereomer (physical separation of the desired diastereomer from the mixture after its crystallization or suspension in step b).
[165]
Claim 3 is a dependant claim that includes the method of Claim 1 and limits it by specifying that the esterifying alcohol in Claim 1a, ie the alcohol R2 OH, is methanol.
[166]
Claim 4 is a dependant claim that includes the method of Claim 1 and limits it by specifying that the tryptophan is the D-tryptophan.
(3)
Construction of Claims 7, 8-10
(a)
The claim in dispute
[167]
Claim 7 is an independent claim, and Claims 8-10, also asserted, are among its dependant claims. Claim 7 is again a method of preparing a compound, and comprising 4 steps:
A method of preparing a compound having a formula:
comprising the steps of:
a) providing a desired diastereomer of a tetrahydro-β-carboline by the method of Claim 1;
b) reacting the tetrahydro-β-carboline with chloroacetyl chloride to provide an N-substituted tetrahydro-β-carboline;
c) reacting the N-substituted tetrahydro-β-carboline with an amine having a structure R3NH2, wherein R3 is C1-6alkyl or hydro; and
d) purifying the compound by recrystallization from glacial acetic acid.
[168]
Claims 8, 9 and 10 are dependant of Claim 7 and are narrower than Claim 7.
(b)
Claim 7 step d
[169]
Claim 7d is where the shoe pinches.
[170]
Lilly submit that Claim 7d, ie the step of purifying, is not an essential element because purification is not essential to the making of tadalafil. Dr. Laird confirms as much and argues his conclusion is supported by the patent’s disclosure at page 26.
[171]
Regarding the use of the prosecution history (file wrapper), Lilly recognize that Canmar is the leading decision on section 53.1 of the Patent Act. However, they take issue with the fact that construction should not be done with the file wrapper, which should merely be used as confirmatory of an already performed construction. Lilly also argue that Dr. Williams’ construction using the file wrapper was improper, as he had not then received representation from Lilly to rebut. Lilly also argue that the whole of the file wrapper must be considered, if part of it were admitted to rebut their representation, so that “reflux temperature or lower”
could be understood as “reflux temperature”
. This would ensure consistency in the application of file wrappers. They argue that they can use it as a sword to sustain their construction once it becomes available to Apotex to rebut their representation.
[172]
Apotex raises section 53.1 of the Patent Act to refer to the file wrapper estoppel. It argues that Lilly’s present argument that Claim 7d is non-essential is precisely contrary to the written communications Lilly made in the course of the prosecution of the 540 Application.
[173]
On April 11, 2008, the Patent Examiner objected to the then Claim 16, on the basis of obviousness under section 28.3 of the Patent Act, in regards to the US 006 Patent (Daugan). On September 9, 2008, Lilly responded and amended its application. It indicated Claim 16 had become Claim 7 and, to overcome the obviousness objection, it incorporated the features of cancelled Claims 19 and 21 into Claim 7 (formerly Claim 16). It specified that the Daugan 006 Patent reference did not teach or suggest a recrystallization step from acetic acid that increases the purity of the compound, as now required by Claim 7 (my emphasis).
[174]
In his decision in Canmar, Justice Manson confirmed that “With the introduction of section 53.1, purposive construction of patent claims in Canada now includes three prongs: (1) the claims themselves; (2) the disclosure; and (3) the prosecution history in Canada, when used to rebut a representation made by the patentee as to the construction of a claim in the patent”
(at para 68). The Court has thus confirmed that the prosecution history can be considered as part of a purposive construction.
[175]
Lilly assert that Claim 7d is non-essential although the applicant added it to the claim and deemed it “required”
in order to alleviate obviousness during its prosecution. As per its clear language, section 53.1 of the Patent Act is used to rebut representations made by the patentee. There is nothing in the text of section 53.1 that allows a patentee to revert to the prosecution history in order to put forward self-serving evidence, and in this case, for Lilly to use it to interpret “reflux temperature or lower”
as “reflux temperature”
.
[176]
I find the 540 Patent’s prosecution history can be raised to rebut Lilly’s representation that Claim 7d is non-essential. It appears clear that Claim 7d is, on the contrary, essential, as the patentee labelled it as “required”
in order to avoid the obviousness finding of the Patent Office.
(c)
Essential elements of Claims 7 and 8-10
[177]
As per my construction, the essential elements of Claim 7 are :
A method of preparing a compound having a formula
comprising the steps of (each steps being essential):
a) providing a desired diastereomer of a tetrahydro-β-carboline by the method of Claim 1;
b) reacting the tetrahydro-β-carboline with chloroacetyl chloride to provide an N-substituted tetrahydro-β-carboline;
c) reacting the N-substituted tetrahydro-β-carboline with an amine having a structure R3NH2, wherein R3 is C1-6aIkyl or hydro; and
d) purifying the compound by recrystallization from glacial acetic acid.
[178]
Claim 8 is a dependant claim that includes the method of Claim 7 and limits it such that the amine in Claim 7c must be selected from the group defined in Claim 8.
[179]
Claim 9 is a dependant claim that includes the method of Claim 7 and limits it such that the amine in Claim 7c must be methylamine.
[180]
Claim 10 is a dependant claim that includes the method of Claim 7 and limits it such that the amine in Claim 7c has an R3 of methyl.
(4)
Construction of Claim 12
(a)
The claim in dispute
[181]
Claim 12 is an independent claim
A method of preparing a compound having a structural formula:
comprising the steps of:
a) esterifying D-tryptophan in methanol and thionyl chloride to provide D-tryptophan methyl ester hydrochloride;
b) reacting the D-tryptophan methyl ester hydrochloride with piperonal in refluxing isopropyl alcohol to provide cis-1-(1,3-benzodioxol-5-y1)-2,3,4,9-tetrahydro 1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester;
c) reacting the product of step (b) with chloroacetyl chloride and triethylamine to provide cis-1-(1,3-benzodioxol-5-y1)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester; and
d) reacting the product of step (c) with methylamine to provide the compound.
[182]
I have again underlined above the passages in dispute between the parties.
(b)
Claim 12 step a and step b: the variants
[183]
Lilly argue that “methanol”
and “thionyl chloride”
in step a, and “isopropryl alcohol”
in step b should be broadened, although they take no issue with the other products mentioned in Claim 12 and do not seek to broaden them.
[184]
Lilly ask that the phrase “or an equivalent variant that would perform substantially the same function in substantially the same way to obtain substantially the same result”
be added to the methanol and thionyl chloride of Claim 12b, and to the isopropyl alcohol of Claim 12c. Once the language is added, Lilly consider the elements as essential.
[185]
Lilly rely on Free World Trust at paras 55–56, examined earlier, pertaining to the determination of essential versus non essential elements, and argue that the Improver approach applies here, thus allowing the patentee to add variants to the elements that are claimed. Lilly, again citing Free World Trust, submit that “It would be unfair to allow a patent monopoly to be breached with impunity by a copycat device that simply switched bells and whistles to escape the literal Claims of the patent”
. They ask the Court to adopt the three part test of Improver in order to determine whether “at the date of publication of the patent, the skilled addressees would have appreciated that a particular element could be substituted without affecting the working of the invention”
(Free World Trust at para 55). If the answer is positive, unless strict compliance with the terms of the invention is inferred from the claims, then its variants could be added to the element claimed, according to Lilly.
[186]
Lilly also point to the UK decision Actavis v Eli Lilly, 2017 UKSC 48 [Actavis], which applied the Improver approach and extended the claims language to not only catch permetrexed disodium, but also equivalent variants that would perform substantially the same function in substantially the same way to obtain substantially the same result. In order to determine whether the variant nonetheless infringes because it varies from the invention in an immaterial way, Actavis slightly reformulated the Improver approach and noted that “if one cannot depart from the language of the Claim […], what is the point of the questions in the first place?”
(at para 71). Although Actavis applied article 69(1) of the European Patent Convention (2000) and the Protocol on the Interpretation of Article 69, Lilly submit that they are no different from Canadian claim construction rules.
[187]
Finally, Lilly ask the Court to reject Dr. Williams’ construction on the matter because he was not given instructions relating to the Improver approach, and to reject Dr. Anderson’s construction because there is no analysis in his report on the applicability of Improver. Lilly thus ask the Court to adopt Dr. Laird’s construction of essential elements on the two parts of Claim 12 as follows:
a) - esterifying D-tryptophan in methanol and thionyl chloride or an equivalent variant that would perform substantially the same function in substantially the same way to obtain substantially the same result, to provide D-tryptophan methyl ester hydrochloride;
b) - reacting the D-tryptophan methyl ester hydrochloride with piperonal in isopropyl alcohol, or an equivalent variant that would perform substantially the same function in substantially the same way to obtain substantially the same result, to provide cis-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester;
[188]
Apotex objects to Lilly’s attempt to add language to the claim. It essentially argues that (1) there is no provision in the Patent Act, nor in any jurisprudence, that supports Lilly’s request; (2) Lilly’s attempt is contrary to subsection 27(4) of the Patent Act, which requires the patentee to define the monopoly in the patent distinctly and in explicit terms; (3) Lilly attempt to engage in the doctrine of equivalence that was rejected by the SCC in Free World Trust at paras 37-40; (4) the narrow specifications was intentional and ought not to be broadened; (5) the language of Claim 12 can be contrasted with that in other claims, which is more general; (6) claim differentiation principle recognizes that, where a patent contains claims that are general and claims that are specific, elements of the specific claims are treated as being essential to that claim. Apotex challenges Dr. Laird’s construction, alleging that he did not consider the patentee’s intention and that his approach is result oriented, as he did not propose adding variants to the trimethylamine in Claim 12c ,despite adding the variants in steps a and b. Apotex thus asks the Court to adhere to the language of the claims and to consider the elements as essential. It also points out that Lilly’s addition of the equivalents to the language of Claim 12 is a misapplication of the SCC’s teachings.
[189]
Lilly refer to para 55 of Free World Trust to advance their position that variants known at the date of publication should be added as essential elements of the claims.
[190]
However, as per the teachings of the SCC in Free world Trust and the subsequent guidance of the FCA in Canamould Extrusions, the task of the Court in claim construction is to determine the elements that are essential and those that are non-essential. This determination will have an impact on the infringement analysis, where the existence of known variants will come into play, as variants of essential elements will fall outside the scope of the monopoly while variants of non-essential elements may be captured by the monopoly.
[191]
In what appears to be somewhat of a circular analysis, the Court can consider factual elements pertaining to the existence of known variants when tasked with qualifying elements as essential or non-essential. However, the patentee’s intention remains the key factor (Free World Trust at paras 58-60; Canamould Extrusions).
[192]
In any event, Lilly bore the burden to demonstrate the elements were non-essential, but they have not satisfied it. I am satisfied that the skilled chemist, at the publication date, would understand from the language of Claim 12a and b, that the patentee intended strict compliance with the primary meaning to be an essential requirement of the invention. If the principles of claim construction are to be respected, the claims should not be construed with the added language at steps a and b: as what is not claimed is disclaimed, each claim must have a meaning, and claim differentiation applies to independent claims.
[193]
The patentee chose to claim particular solvents, knowing others could work, and made its bed by limiting Claim 12 to the named elements. Claim 1, which overlaps with Claim 12, is specifically worded in a more open manner, and the skilled chemist would understand the patentee intended to draw a narrower monopoly with Claim 12. Dr. Laird did not explain why variants should be added only to Claim 12a and Claim 12b, but not to Claim 12c, thus leaving open the possibility that he construed the claim with a result in mind, which is not permitted because claim construction is antecedent to invalidity and infringement.
[194]
Finally, the disclosure’s last paragraph also runs counter Lilly’s position as it states that “many modifications and variations of the invention as set forth above can be made without departing from the spirit and scope thereof, and, therefore, only such limitations should be imposed as are indicated by the appended claims”
. As words bring limitations, the skilled reader would understand the patentee’s intention to limit the choice of solvents to those named although others may work.
[195]
So, Claim 12a and b stand as they are, the named solvents are essential elements, and the variants sought by Lilly are not added as additional essential elements of the Claim.
(c)
Claim 12 step c
[196]
Lilly recognise there is an error in Claim 12c, as the chemical name that is written is not the product of the reaction in c, but the repetition of the product in Claim 12b. The chemical name of Claim 12c should read instead “chloroacetyl tetrahydro-β-carboline”
.
[197]
However, Lilly confirm they are not asking the Court to rewrite the element of Claim 12 as it relates to the misnaming of the compound provided in Claim 12c or to correct it. They are asking the Court to simply accept the evidence of the experts providing how a skilled person would read Claim 12c. Essentially, Lilly argue that the skilled person understands the error and accordingly understands the scope of the claim, which accords with the purposive approach construction. The PSA would understand that the product of step b, after it is reacted with chloroacetyl chloride and triethylamine, would be chloroacetyl tetrahydro-β-carboline. Dr. Laird also indicated the reaction to be at page 23 of the 540 Patent.
[198]
Apotex argues that the Court should not correct the error and should refrain from rewriting Claim 12c, which it alleges, will impact the utility assessment.
[199]
As this is the section of the decision pertaining to claim construction, and as the parties agree that the Court should not rewrite Claim 12c but leave it as it stands, this is what I will do.
[200]
Whether this has an impact on the assessment of the allegation of invalidity on the ground of inutility/inoperability raised by Apotex will be examined further later in these reasons.
(d)
Essential elements of Claim 12
[201]
As per my construction, the essential elements of Claim 12 are:
A method of preparing a compound having a structural formula:
comprising the steps of (each step being essential):
a) esterifying D-tryptophan in methanol and thionyl chloride to provide D-tryptophan methyl ester hydrochloride;
b) reacting the D-tryptophan methyl ester hydrochloride with piperonal in refluxing isopropyl alcohol to provide cis-1-(1,3-benzodioxol-5-y1)-2,3,4,9-tetrahydro 1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester;
c) reacting the product of step (b) with chloroacetyl chloride and triethylamine to provide cis-1-(1,3-benzodioxol-5-y1)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester; and
d) reacting the product of step (c) with methylamine to provide the compound.
VI.
Apotex’s counterclaim of invalidity
A.
Introduction
[202]
As outlined in subsection 43(2) of the Patent Act, a patent is presumed to be valid. It is Apotex’s burden to prove invalidity on the balance of probabilities.
[203]
Apotex raises four grounds of invalidity, anticipation of Claims 1, 3 and 4 by the 594 Application, obviousness of all the asserted Claims given the 594 Application and the 377 Patent (US 006 Patent), inutility of Claim 12 because of the error in Claim 12c, and overbreadth of Claim 1 because ethyl acetate is a listed solvent in the disclosure, but does not work as promised.
[204]
In brief, and for the reasons exposed hereinafter I find Apotex has established anticipation of Claims 1, 3-4 and obviousness of the asserted Claims, but has established neither inutility of Claim 12 nor overbreadth of Claim 1.
B.
Anticipation
(1)
The anticipation allegations
[205]
Apotex alleges that Claims 1, 3 and 4 of the 540 Patent are invalid for anticipation. As required in an allegation of invalidity for anticipation, Apotex points to one element of prior art, the 594 Application, published on January 3, 2002, alleging that it discloses and enables the essential elements of Claims 1, 3 and 4.
[206]
Apotex argues that, although the 540 Patent discloses two prior art methods in an effort to distinguish the patented method from the prior art, it fails to compare its invention with another prior art found in the 594 Application.
[207]
Stating that “what infringes if later, anticipates if earlier”
, (Sanofi at paras 23–27; Abbott Laboratoire v Ratiopharm; Abbott Laboratories v Canada (Health), 2006 FCA 187), Apotex argues that the essential elements of Claims 1, 3 and 4 of the 540 Patent are anticipated by the process outlined to prepare the Intermediate 1 of Example 2, particularly the preparation set out at page 34 of the 594 Application. Apotex outlines that this process represents a 100gram scale synthesis of pure cis-diastereomer (59% yield), a target compound of Claims 1, 3 and 4 of the 540 Patent, and that the description of the example in the 594 Application reads directly on all the essential elements of Claims 1, 3 and 4 as (1) it starts with D-tryptophan methyl ester hydrochloride (ie the tryotophan ester is provided); (2) it reacts D-tryptophan methyl ester hydrochloride with piperonal to provide the cis and trans-diastereomers and the reaction is run in acetic acid with water, a solvent mixture that is acknowledged by the patent to be a solvent in which the cis-diastereomer is insoluble relative to the trans-diastereomer; and (3) the cis-diastereomer is separated from the dissolved trans-diastereomer by filtration.
[208]
Apotex presents each step of Claim 1, referenced in Claims 3 and 4, and argues that they are all anticipated by the 594 Application.
[209]
In regards to Claim 1a, Apotex stresses that Dr. Laird, Lilly’s expert, confirmed that it was present in the 594 Application (Dr. Laird Validity Report at page 25).
[210]
In regards to Claim 1b, Apotex outlines that Dr. Laird conceded the solvent used in the 594 Application was an aqueous solution of acidic acid and water of the type explicitly described in the 540 Patent. Apotex argues Dr. Laird also conceded that the 540 Patent teaches the PSA that the reaction can also be run with toluene, ethyl acetate, mixtures of these solvents, aqueous solutions, acetronile and water, toluene and acetronitrile, and acetic acid and water. After reviewing the preparation, Apotex stresses that, at the 73th hour, a “resulting suspension”
was observed, which both Dr. Anderson and Dr. Williams opined was the desired cis-diastereomer.
[211]
In regards to Claim 1c, Apotex submits that the 594 Application provides that “the solid was collected by filtration”
(Dr. Anderson Expert Validity Report at para 160; transcript of January 23, 2020 at page 99).
[212]
Lilly do not dispute that the 594 Application is a piece of prior art for the purposes of the anticipation analysis under section 28.2 of the Patent Act. They respond however, that Apotex has not met its burden with respect to anticipation.
[213]
In regards to Claim 1a, Lilly assert that Dr. Anderson admitted that it was not specifically disclosed in the 594 Application, although their own expert, Dr. Laird, confirmed it was present in the 594 Application.
[214]
In regards to Claim 1b, Lilly indicate that the starting material are not in dispute. However, they add that the solvent of the 594 Application, ie the acetic acid, was not known by the PSA to be a solvent where the desired diastereomer is insoluble and the undesired diastereomer is soluble. Furthermore, the PSA would not know about the nature of the suspension, and the suspension needed to be cooled and anti solvents added, which can be contrasted with the 540 Patent. Without knowledge if what is in that suspension, Lilly assert that Apotex has not met its burden to prove that there has been disclosure. Lilly argues that both Drs. Laird and Anderson agree that Claim 1a and Claim 1b are not disclosed, while Dr. Laird adds that Claim 1c, which he construed as a phase separation, is also not disclosed by the 594 Application.
[215]
The main dispute as between the parties thus lies in Claim 1b, as to whether or not the suspended solid observed at the end of the reaction of the 594 Application is the desired cis-diastereomer. Dr. Anderson is adamant it is, while Dr. Laird assumes it is not. Lilly argue that the “flag has not been planted by the 594”
because we don’t know what the suspension is. Lilly assert that Apotex could have conducted experiments to reproduce Exemple 2 of the 594 Application to confirm what the suspension is, but they did not and do not have this information. Dr. Laird was steadfast that all that exist at the end of Intermediate I is a suspension that is not identified. Without knowledge of this suspension, Lilly argue there can be no enablement.
[216]
Apotex’ anticipation attack is aimed at Claim 1, its method being referenced in Claims 3 and 4. Hence, only Claim 1 is measured against the 594 Application. In fact, the 594 Application exemplifies a process using D-tryptophan as in Claim 4 of the 540 Patent, and the fact that Claim 3 narrows Claim 1a has no effect on the analysis as the parties disagree only on whether the whole of the esterification reaction is disclosed and not on whether methanol is used for the reaction.
(2)
The anticipation framework
(a)
Section 28.2 of the Patent Act and the Sanofi test
[217]
The anticipation allegations are governed by section 28.2 of the Patent Act. Under the heading “Subject-matter of claim must not be previously disclosed”
, section 28.2 states that the subject-matter defined by a claim in an application for a patent in Canada (the “pending application”
) must not have been disclosed. It appears clear that the disclosure step of the anticipation test in embedded in the words of the statute.
[218]
The parties agree that the anticipation test is laid out in Sanofi and that, in order to determine if a subject-matter is anticipated, a two steps claim by claim analysis must be performed. The first step is a requirement of prior disclosure and means that the prior art, as of the relevant date, must disclose subject-matter, which if performed, would necessarily result in an infringement of the patent (Sanofi at para 25). If yes, the second step is to look at enablement and ask whether a PSA would have been able to perform the invention (Sanofi at para 26). The enablement must come from a disclosed single prior art reference (Beloit at 297) such that the PSA can “perform or make the invention of the second patent without undue burden”
(Sanofi at para 33). The PSA may apply common general knowledge in the assessment of enablement (Sanofi at para 37). If trials and experiments are generally carried out, the threshold for undue burden will tend to be higher than in circumstances in which less effort is normal. Furthermore, routine trials should not be considered as being undue burden (Sanofi at para 37).
[219]
The anticipation analysis must be made as of the claim date, July 31, 2002.
(b)
The disclosure requirement
[220]
It is not disputed that the subject-matter defined by a claim correspond here to the essential elements of the claim as construed.
[221]
As detailed below, both parties executed their disclosure analysis by outlining the essential elements of Claim 1 of the 540 Patent, and by examining, one by one, if the 594 Application disclosed each of them.
[222]
Dr. Laird, Lilly’s expert, conducted his analysis in this manner (Table 1 of Dr. Laird’s Validity Report). I note that, although Dr. Laird implicitly referred to the result of the PSR (ie, the interconversion of the trans-diastereomer to cis, CIAT process in his Table 1, by referring to paras 76-82 of his report), his analysis and conclusion on anticipation concerned only the essential elements of Claim 1 (Dr. Laird Validity Report at para 86).
[223]
In this component of the trial, Lilly did not suggest the disclosure analysis should proceed to examine if each element of the prior art, if performed, would infringe the claims of the patent in suit, position they asserted in the component of the trial pertaining to the 684 Patent, for selection and non-selection patents alike. Dr. Laird, Lilly’s expert, did not here first outline the 594 Application to examine if all its elements were found in the 540 Patent.
[224]
I find the principle of the disclosure requirement in this component of the trial, followed by both parties, to be the proper one.
(i)
Claim 1 step a (referenced in Claims 3 and 4)
[225]
Dr. Laird confirmed, at page 25 of his Validity Report, that Claim 1a of the 540 Patent is present in the 594 Application.
[226]
Dr. Anderson, in his Expert Report, noted that the 594 Application commences its synthesis with D-tryptophan methyl esther hydrochloride salt, opting to purchase this compound rather than prepare it via a conventional esterification reaction with methanol. Dr. Anderson added that this type of esterification reaction is a basic reaction that is taught to the PSA in an undergraduate setting, and that the PSA would be well prepared to conduct an esterification reaction to produce this compound using knowledge from their general training. He pointed out that, while the PSA cannot be certain, given that the ester was purchased commercially, he or she would expect that the esterification was carried out with an alcohol. He opined therefore that the Claim 1a was generally disclosed, but not specifically disclosed (Expert report of Neal Anderson paras 152–153).
[227]
I find it compelling that Lilly’s expert, Dr. Laird, opined without ambiguity that Claim 1a is disclosed by the 594 Application, while Dr. Anderson cautiously opined it was generally disclosed. Dr. Anderson’s prudent approach does not displace Dr. Laird’s and I consequently find that Apotex has met its burden to establish that Claim 1a, referenced in Claims 3 and 4, is disclosed by the 594 Application.
(ii)
Claim 1 step b (referenced in Claims 3 and 4)
[228]
Claim 1b requires that the reaction be performed in a solvent in which the desired diastereomer is insoluble at reflux temperature or lower, and the undesired diastereomer is soluble at reflux temperature or lower.
[229]
As for the first contentious issue, Dr. Laird confirmed that acetic acid and water is an aqueous solution of the sort explicitly described on page 22 of the 540 Patent, and is the solvent disclosed in the 594 Application.
[230]
The contention of this Claim 1b rests on the identity of the suspension at the 73rd hour mark: is this the insoluble desired cis-diastereomer or is it something else?
[231]
Apotex argues that it can only be the desired cis-diastereomer, and nothing else. For Apotex, it defies logic that the resulting suspension can be anything but the desired cis-diastereomer. Apotex points to Dr. Anderson’s testimony, when he opined to the Court that “So I believe that it is most likely, highly likely, almost certainly the case […] the suspended solid […] at the 73-hour mark were indeed the desired cis-diastereomer”
(transcript of January 20, 2020 at page 47; transcript of February 7, 2020 at page 133) and to Dr. Williams’ opinion.
[232]
For Apotex, should the suspension be anything but the desired product, it would mean that the solid collected at the end is different from the suspension at the 73rd hour. After the cooling and the addition of ethyl acetate and MTBE, which Apotex experts testified as being commonly known anti-solvents (transcript of January 20, 2020 at page 33), the reaction is slowed down to a negligible speed (transcript of January 24, 2020 at page 32). The anti-solvents were added to coax more of the desired product to precipitate (Dr. Anderson Expert Validity Report at para 155). If the suspension at the 73rd hour was not the one desired, Dr. Anderson indicated that it would be logical to remove the suspension, which would have been easy, but this was not done. Also, if the suspension at the 73rd hour was trans, ethyl acetate and MTBE would have to first dissolve the suspension and coax out cis solid, which is overly convoluted and unlikely (Dr. Anderson Reply Report at para 33; transcript of January 20, 2020 at page 38). Dr. Anderson testified that the suspension could not be starting material because the fact that the solution was clear after 24 hours means that D-tryptophan methyl ester hydrochloride can dissolve in acetic acid (transcript of January 20, 2020 at page 37). Combined with the fact that toward the end of the reaction, there was inevitably less D-tryptophan methyl ester hydrochloride remaining in solution, this would enable further dissolution, which would preclude the resurfacing of starting material as a suspension according to Dr. Anderson (transcript of January 20, 2020 at page 37). Also, Dr. Anderson added that if after the second charge, there was non dissolved D-tryptophan methyl ester hydrochloride, the patentee would have left a note similar as after the first charge (transcript of January 20, 2020 at page 35; Reply Expert Report of Dr. Anderson at para 30).
[233]
Apotex thus submits that a PSA on a normal reading of the 594 Application would understand that the suspension is the desired cis-diastereomer.
[234]
Lilly argue, on the contrary, via Dr. Laird, that the 594 Application does not disclose a PSR reaction in which the desired cis-diastereomer is insoluble in the solvent at reaction temperature (Dr. Laird Validity Report at para 76) and the suspended material noted in the 594 Application may not be the desired cis-diastereomer: it could be trans, could be starting material or could be a mixture (Dr. Laird Validity Report at para 80). As well, there is no indication of whether this is a thin or thick suspension. Lilly also argue that Dr. Williams admitted, prior to his corrections in the witness stand, that the crystallization happened with the addition of anti-solvents. As such, Lilly submit that, in the 594 Application, the desired cis-diastereomer is insoluble only after the addition of anti-solvents. Lilly also cite Dr. Anderson on cross-examination, who testified that, in principle, the suspension could contain some of the trans-diastereomers.
[235]
I accept Dr. Anderson’s opinion that the skilled process chemist, reading the 594 Application, would understand that the resulting suspension to be almost entirely composed of the cis-diastereomer. Dr. Anderson’s argument on the nature of the resulting suspension is convincing, that this suspension cannot be the starting material nor should it contain the trans-diastereomer in any significant amount, due to the purity of the solid collected shortly at the end of the process.
[236]
Lilly have not convinced me it would be proper to require Apotex to conduct an experiment to determine the nature of the suspension as part of the first part of the anticipation analysis (Sanofi at para 32). Having favored Dr. Anderson’s opinion, I conclude it is more probable than not that the cis-diastereomer is insoluble, whereas the trans is soluble, in the mixture at the reaction temperature, leading to a phase separation within the mixture, and that the suspension of the 73rd hour is the desired cis-diastereomer.
[237]
The 594 Application therefore discloses Claim 1b of the 540 Patent, referenced in Claims 3 and 4.
(iii)
Claim 1 step c (referenced in Claims 3 and 4)
[238]
Neither party truly submitted arguments regarding Claim 1c as construed by the Court. It appears clear the addition of anti-solvents, the cooling, and the final filtration of the solid of the 594 Application constitute a separating step of the diastereomer from the mixture, which is how I have construed Claim 1c. Dr. Anderson opined at paragraph 160 of his Validity Report that “Intermediate 1 was isolated from the reaction mixture via filtration”
and that this is “an example of physical separation of the two diastereomeric products of this Pictet-Spengler reaction.”
[239]
I find the 594 Application discloses Claim 1c of the 540 Patent, referenced in Claims 3 and 4.
(iv)
Conclusion on disclosure
[240]
Example 2 of the 594 Application discloses all the essential elements of Claim 1 of the 540 Patent. Claim 4 is also disclosed because of the use of commercially provided D-tryptophan methyl ester hydrochloride in the process outlined in the 594 Application. Claim 3 is also disclosed as the parties disagree only on whether the whole of the esterification reaction is disclosed and not on whether methanol is used for the reaction, and having taken into account Dr. Laird’s admission, I have concluded that Claim 1a is disclosed, so is Claim 3.
[241]
The subject-matter identified in the prior art, if performed, would result in an infringement of Claim 1 of the 540 Patent.
(c)
The enablement requirement
[242]
Apotex contends that the process outlined on Intermediate 1 of Example 2 at page 34 of the 594 Application is sufficiently detailed and includes the identity and amount of reagents, temperatures, etc. such that a PSA would easily be able to follow it like a cookbook recipe, which is all what is required for enablement of Claims 1, referenced in Claims 3 and 4 of the 540 Patent.
[243]
On the contrary, Lilly argue that the esterification reaction was not disclosed, which precludes enablement, and that their expert, Dr. Laird, was steadfast in his opinion that the 594 Application does not enable Claim 1 of the 540 Patent. All that exists at the end of the PSR in the 594 Application is a suspension of indeterminate composition that could be unreacted starting material or by product or a mixture of the cis and trans-diastereomer, without being told what it is.
[244]
For enablement, a PSA, following the prior art, must be able to arrive at the invention claimed in the impugned patent, which is in this case only Claims 1, 3–4, without undue burden (Sanofi at para 43).
[245]
Given my conclusions on the esterification with methanol reaction and on the nature of the suspension, and as I adopted Dr. Anderson’s opinion that the 594 Application’s suspension is the cis-diastereomer, Claims 1, 3 and 4 are enabled by the 594 Application.
(3)
Conclusion on anticipation
[246]
Apotex has established that Claims 1, 3 and 4 of the 540 Patent are disclosed and enabled by the 594 Application, and are thus anticipated. They are, therefore, invalid.
C.
Obviousness
(1)
The obviousness allegations
[247]
Apotex alleges that all of the asserted Claims of the 540 Patent are obvious to the PSA, as of July 31, 2002, and are thus invalid. Although Lilly deny the obviousness of all the claims, the main contentious issues and arguments of the parties center on the PSR of Claim 1b and Claim 12b.
[248]
Apotex submits that the subject-matter defined by a claim stated in section 28.3 of the Patent Act lies in the essential elements of the asserted Claims, not in randomly selected elements from the disclosure. It adds that the obviousness test in Sanofi, to the extent that it allows the parsing of the patent’s disclosure, is only applicable to selection patents and patents filed before October 1, 1989 that are not governed by section 28.3 of the Patent Act.
[249]
Hence, Apotex alleges that the PSR of Claim 1b was taught by the 594 Application published on January 3, 2002 and was further taught by the 377 Patent issued in May 28, 2002. In regards to Claim 7d, Apotex submits that the PSA would have no problem identifying the proper solvent based on differential solubility to recrystallize and purify tadalafil. With respect to the PSR of Claim 12b, Apotex submits that the 540 Patent itself admits that the selection of a particular solvent, in this case, isopropyl alcohol is well within the ability of the PSA, and that this admission renders Claim 12 obvious in light of the 594 Application and of the 377 Patent.
[250]
Lilly do not contest the 594 Application and the 377 Patent as prior art, but respond the 540 Patent is not obvious. Lilly submit that the inventive concept provided in the Sanofi test, hence the subject-matter defined by a claim of section 28.3 of the Patent Act, is different from the claim construction. They add that in this case, the inventive concept includes the CIAT process, ie the results or benefits of the modified PSR, leading to equilibration, high yield, high purity, faster process and less steps.
[251]
Lilly rely on Dr. Laird’s opinion that the inventive concept of the claims of the 540 Patent is “the design of an improved PSR that takes place in a solvent in which the desired cis-diastereomer is insoluble at reflux temperature (or lower) and the undesired trans-diastereomer is soluble at reflux temperature (or lower). This results in the simultaneous conversion, due to equilibration, of the soluble trans-diastereomer into the cis during the PSR, resulting in high yield and high purity of the cis-diastereomer and short processing times”
(Dr. Laird Validity Report at para 95). Lilly thus argue that the effect of the PSR cannot be disregarded when considering the second point in the inventiveness analysis, and cite Teva Canada Limted v Pfizer Canada Inc, 2019 FCA 15 at para 35 and Apotex Inc v Pfizer Canada Inc, 2019 FCA 16 at para 39.
[252]
Lilly argue that, under the obviousness analysis, the subject-matter defined by a claim, ie the inventive concept, must be construed and that it can differ from the essential elements of the claims as construed. The inventive concept, as per Lilly’s submissions, can thus include the result, the benefit or the effect of the invention. Lilly submit that it is indeed arguable, from Sanofi, that the inventive concept is different from the claim construction.
[253]
Given the parties’ contradictory positions, I must determine how the SCC and the FCA have directed this Court to conduct the obviousness enquiry.
(2)
The obviousness framework
(a)
Section 28.3 of the Patent Act
[254]
The obviousness assessment is governed by section 28.3 of the Patent Act, which states that the subject-matter defined by a claim in an application for a patent in Canada must be subject-matter that would not have been obvious.
[255]
In this case, the 540 Patent must not have been obvious for the PSA on July 31, 2002.
(b)
The Sanofi test on obviousness
[256]
In 2008, the SCC issued its decision in Sanofi, recognised since as the seminal decision on the obviousness inquiry. Sanofi pertained to a selection patent, and, as it was not governed by section 28.3 of the Patent Act, it was not discussed.
[257]
In Sanofi, finding the test of obviousness in Beloit too rigid, the SCC indicated it would be useful to follow the four-step approach first outlined in Windsurfing International Inc v Tabur Marine (Great Britain) Ltd, [1985] RPC 59 (EWCA) [Windsurfing] and updated in Pozzoli SPA v BDMO SA [2007] EWCA Civ 588 [Pozzoli]. The SCC restated the Windsurfing questions, at para 67, as:
1) (a) Identify the notional “person skilled in the art”;
(b) Identify the relevant common general knowledge of that person;
2) Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
3) Identify what, if any, differences exist between the matter cited as forming part of the “state of the art” and the inventive concept of the claim or the claim as construed;
3) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?
[258]
In regards to the inventive concept, the SCC found it was not readily discernable from the claims themselves, and thus referred to the rest of the specification to identify it: “A bare chemical formula in a patent claim may not be sufficient to determine its inventiveness. In such cases, I think it must be acceptable to read the specification in the patent to determine the inventive concept of the claims”
(Sanofi at para 77). The SCC found the inventive concept of the claims of the selection patent in suit to reside in its advantages over the other compounds of its genus patent and in the methods for obtaining that compound (at para 78).
[259]
I will examine each step.
(c)
First step: identify the notional PSA and the relevant common general knowledge of that person
[260]
The PSA has already been identified by the Court at paras 114-115, and the common general knowledge has also been identified at paras 128-134.
(d)
Second step: identify the inventive concept of the claim in question or if that cannot readily be done, construe it
(i)
Issues
[261]
Following Sanofi, much debate have ensued to determine if, by introducing the term “inventive concept”
in the obviousness test and by referring to the disclosure to construe it, the SCC in fact changed the jurisprudence that prevailed, set by the FCA in Beloit. Questions arose as to the meaning of “inventive concept”,
whether it is different from the claim construction, whether the test set by the SCC under former provisions of the Patent Act applied to patents governed by section 28.3 of the Patent Act, or whether it is permitted to ascertain the inventive concept from outside the claims in the context of selection, or non-selection patents, if it is, or not, readily discernible from the claims. As the parties disagree on how to answer these questions, I will briefly expose the jurisprudence and the legislative amendment chronologically, situate Sanofi in that chronology, and outline the answers the FCA provided up to now to direct my analysis.
(ii)
1986: the Beloit framework
[262]
Prior to 1993, the Patent Act contained no specific provision on obviousness, or its antithesis: inventive ingenuity and inventiveness. Inventiveness, as a requirement for patentability, was ingrained within the definition of the word invention of section 2 of the Patent Act.
[263]
Until 2008, the leading case on obviousness was the FCA’s decision in Beloit, where in fact, both parties had obtained a patent for the same invention. The patent related to a press mechanism installed on one of the four sections of a paper machine, and it was not a selection patent.
[264]
Justice Hugessen, for the FCA, indicated that what was claimed as novel and inventive was the combination of previously known elements in the design of a high-speed press section, and outlined a simplification and vulgarisation of the patent’s claim and the actual text of the claim, which did not mention the speed of the machine.
[265]
Justice Hugessen confirmed the proper test for obviousness. He first stated that the test was not to ask what competent inventors would have done, as inventors are by definition inventive. He added that the “classical touchstone for obviousness is the technician skilled in the art but having no scintilla of inventiveness or imagination; a paragon of deduction and dexterity, wholly devoid of intuition; a triumph of the left hemisphere over the right”
and that the question to be asked was “whether this mythical creature [the man in the Clapham omnibus of patent law] would in light of the state of the art, and of common general knowledge as at the claimed date of invention, have come directly and without difficulty to the solution taught by the patent. It is a very difficult test to satisfy”
(Beloit at 294, my emphasis).
[266]
Justice Hugessen thus referred to “the solution taught by the patent”
as the element that must be compared to the prior art, ie as the second point, but he did not define it. As we will see below, the solution taught by the patent was later interpreted to mean the claim or claims as construed by the Court.
[267]
Justice Hugessen identified a series of ascertainable facts as to which there was no dispute, and found that their cumulative effect showed inventiveness: (1) the defendant in that case had claimed and continued to claim inventiveness for the same apparatus; (2) the speed of the machine increased; (3) it was difficult in getting the new machine accepted because convention wisdom pointed away from the invention; and (4) the machine was an outstanding commercial success after its acceptance.
(iii)
Section 28.3 of the Patent Act
[268]
In 1993, the Patent Act was modified, and section 28.3 was introduced, applying to patent applications filed on or after October 1, 1989. It identified the second point, ie the element that must be compared against the prior art, as the subject-matter defined by a claim. As we examined earlier, it is the same term as the one used in section 28.2, governing the anticipation enquiry.
[269]
In Janssen-Ortho Inc v Novopharm Ltd, 2006 FC 1234, although the patent in suit was not governed by section 28.3 of the Patent Act, Justice Hughes noted the legislative change and the fact that a definition of obviousness had been introduced. Justice Hughes stated that the definition “is not different from the law as it was generally understood previously”
(at para 109). He questioned whether the solution taught by the patent, ie the invention taught, was different from the claim as properly construed. He confirmed the test for obviousness was that of Beloit, and confirmed that what was at issue is the claim or are the claims as construed by the Court: the “invention as generally expressed in the patent or by the inventors is not the issue, it is the claim as properly construed”
(at para 113). He discussed a list of factors and noted primary and secondary ones. He determined the invention as claimed to be the claim as he construed, which did not include the compound’s properties or uses (at para 114). The FCA upheld Justice Hughes’s decision in 2007 FCA 217 (Janssen FCA), and confirmed the test set by Beloit in that “what it in issue is the patent claim as construed by the Court”
(at para 25).
[270]
From Janssen FCA, it appears clear that the solution taught by the patent, as the second point set out by Beloit, was understood to be equivalent to the claims as construed by the Court.
(iv)
Sanofi in 2008
[271]
As mentioned earlier, in 2008, the SCC examined the obviousness framework in the context of a selection patent, not governed by section 28.3 of the Patent Act. The SCC introduced the term “inventive concept”
to designate the “second point”
, ie, the element that must be compared to the prior art, and what the FCA in Beloit referred to as the “solution taught by the patent”
. Noting that the inventive concept was not discernable from the claims, the SCC referred to the disclosure to identify it as “a compound useful in inhibiting platelet aggregation which has greater therapeutic effect and less toxicity than the other compounds of the 875 patent and the methods for obtaining that compound”
(Sanofi at paras 77–78).
(v)
Post-Sanofi
[272]
Shortly after Sanofi, the FCA issued its decision in Apotex Inc v ADIR, 2009 FCA 222 [ADIR FCA]. The FCA then reviewed a decision rendered by the FC before Sanofi was issued, in which Justice Snider had applied the framework set out in Janssen FCA. The patent in suit was not a selection and was not governed by section 28.3 of the Patent Act.
[273]
Before the FCA, Apotex argued that the trial judge had erred by directing the obviousness inquiry to the claims of the patent and rejecting what the disclosure taught about inventiveness. The FCA found the framework in Janssen FCA was not inconsistent with what was described in Sanofi. It rejected Apotex’s proposition by endorsing and adopting the reference from Conor MedSystems Inc v. Angiotech Pharmaceuticals Inc. [2008] UKHL 49 [Conor MedSystems] at para 19: “the invention is the product specified in a claim and the patentee is entitled to have the question of obviousness determined by reference to his claim and not to some vague paraphrase based upon the extent of his disclosure in the description”
. The FCA cited Janssen FCA for the proposition that “what is in issue is the patent claim as construed by the Court” and reconciled its statement with Sanofi, noting that Justice Rothstein had stated the second step to be the need to identify the inventive concept of the claim in question or if that cannot readily be done construe it”
(ADIR FCA at para 69)).
[274]
In Novopharm FCA, Justice Layden-Stevenson endorsed the framework set out in Sanofi to assess the obviousness allegations in regards to a selection patent, governed by section 28.3 of the Patent Act.
[275]
Justice Layden-Stevenson, before addressing the obviousness analysis, stated there was no authority where the analysis of the conditions for a valid selection patent, without more, rendered a patent invalid. She confirmed that a selection patent is the same as any other patent and its validity is vulnerable to attack on any of the grounds set out in the Patent Act. However, she added that the conditions for a valid selection patent serve to characterize the patent and accordingly inform the analysis for the grounds of validity set out in the act (at para 27). In regards to obviousness, Justice Layden-Stevenson confirmed that “in the context of a selection patent, the obviousness analysis considers the special properties of the compound, along with its alleged advantages, as described in the selection patent disclosure, for it is there that the inventiveness of the selection lies”
(my emphasis). Justice Layden-Stevenson did not indicate or discuss how the obviousness inquiry of a patent that is not a selection should be informed. There is thus no indication that she displaced the teaching of the FCA in ADIR FCA for patents that are not selections.
[276]
However, other decisions adopted a different position, such as Allergan Inc v Canada (Health), 2011 FC 1316 at paras 53–54 [Allergan]; Apotex Inc v Allergan Inc 2012 FCA 308; Bell Helicopter Textron, creating somewhat of a confusion.
[277]
The parties have particularly outlined five recent decisions of the FCA shedding light on the interpretation and the application of the Sanofi obviousness test, and I will thus examine them briefly to identify the interpretation the FCA directs me to adopt. These decisions pertain to patents that are not selections, and are governed by section 28.3 of the Patent Act.
[278]
In Zero Spill Systems (Int’l) Inc v Heide, 2015 FCA 115 [Zero Spill FCA], Justice Stratas confirmed that sections 28.2 and 28.3 of the Patent Act both begin with the same terms, and that both require a reviewing court to focus on the subject-matter “defined by a claim”
(at para 81). Justice Stratas noted sections 28.2 and 28.3 established a standard, and certain conditions for their application, but they did not prescribed a test. He confirmed that the leading authority on anticipation and obviousness was the Sanofi decision (although it was decided under the former version of the Patent Act) which “affirmed two common law tests, each of which confirms that invalidity for anticipation or obviousness must be established claim by claim”
(at para 85). I have not found in Justice Stratas’ reasons a clear definition of Sanofi’s “inventive concept”, nor reference that the inventive concept of Sanofi derives from a common law test
.
[279]
In Bristol-Myers Squibb Canada Co v Teva Canada Limited, 2017 FCA 76 [BMS FCA], Justice Pelletier examined the obviousness framework. Commenting on the Sanofi decision, he wrote that its innovative feature, in relation to obviousness, was its adoption of the “obvious to try”
test linked to UK jurisprudence of Windsurfing/Pozzoli and the three Lundbeck “obvious to try”
factors (H. Lundbeck A/S v Generics (UK) Ltd, [2008] EWCA Civ. 311).
[280]
In regards to the inventive concept, Justice Pelletier, in BMS FCA, outlined the fact that the SCC had not discussed its reasons for adopting the Windsurfing/Pozzoli framework. He also noted that the SCC had not referred to the cautionary note struck in Pozzoli regarding the inventive concept to the effect that “in the end what matters is-are the difference(s) between what is claimed and the prior art”
(at para 63). Justice Pelletier added that, until Sanofi, the jurisprudence followed Beloit and referred to “the solution taught by the patent”
, and that, since Sanofi, varying interpretations of the inventive concept had been applied. He also remarked that the SCC in Sanofi modified the test for obviousness by modifying the manner in which the gap between the prior art and the solution taught by the patent can be bridged, but did not, without saying so, change the definition of obviousness (BMS FCA at paras 67–68). He determined that the SCC’s use of the term “inventive concept”
had not changed what the prior art must be compared against, and ultimately found the Federal Court erred by implicitly adopting a definition of the inventive concept which focused on the properties of the compounds (at para 74). The inventive concept amounts to what is claimed in the patent.
[281]
In Ciba FCA, Justice Pelletier again examined the obviousness test, and the meaning of the term inventive concept as found in Sanofi. He cited Unilever v Chefaro, [1994] RPC 567 (Pt Ct), and Conor MedSystems for the proposition that the “patentee is entitled to have the question of obviousness determined by reference to his claim and not to some vague paraphrase based upon the extent of his disclosure in the description”
. He held that this focus on the claims is consistent with section 28.3, which stipulates that it is the subject-matter defined by a claim, which must not be obvious. He stressed that the term inventive concept remained undefined, which brought considerable confusion, and suggested we avoid it altogether until the SCC is able to develop a workable definition. Justice Pelletier then proceeded to compare the prior art with the elements of the claims as construed.
[282]
In Tearlab FCA, the discussion of the inventive concept in regards to the obviousness inquiry starts at para 75 of the decision. Justice de Montigny cited Sanofi and commented that the SCC has hinted claim construction and inventive concept are not identical concepts, although it offered no description or explanation as to what inventive concept actually is, leaving many to wonder if they are, in practice, different. Justice de Montigny cited BMS FCA for the proposition that references in the jurisprudence to “the inventive concept”
, “the solution taught by the patent”
, or simply “the invention”
, are merely attempts to define the second point, and are treated as synonymous with “what is claimed”
in the patent (at para 77).
[283]
Justice de Montigny then referred to recent decisions of the FCA (Ciba FCA and ADIR FCA) that have downplayed the importance of the “inventive concept”
as an analytical tool in the context of an obviousness analysis, and focused the analysis on the claims themselves, in line with the principle expressed by Lord Hoffmann in Connor at para 19 (Tearlab FCA at para 78).
[284]
In Hospira FCA, Justice Locke confirmed the reference to section 28.3 as the statutory basis for a requirement of inventiveness as well as the four step approach to obviousness analysis as set out in para 67 of Sanofi. Justice Locke also focused on the claims in ascertaining the inventive concept by reiterating the principle that “the claimed invention for any given claim in issue is defined by the essential elements thereof, which do not contemplate any particular experiments or results”
(at para 94).
[285]
In addition, I wish to point out that in 2017, the SCC issued its decision in AstraZeneca SCC, abolishing the promise doctrine. Non-obviousness was not in issue and the SCC did not address it, save the mention at para 31 that “Generally, an analysis regarding issues of validity, such as novelty or non-obviousness, focuses on the claims alone, and only considers the disclosure where there is ambiguity in the claims (Sanofi-Synthelabo). This is in accordance with this Court’s direction that claims construction precedes all considerations of validity: Free World Trust v. Électro Santé Inc., 2000 SCC 66, [2000] 2 S.C.R. 1024, at paras. 33-50; Whirlpool Corp. v. Camco Inc., 2000 SCC 67, [2000] 2 S.C.R. 1067, at paras. 42-43.”
[286]
Obviousness was, however, an issue in the Federal Court’s decision (AstraZeneca Canada Inc v Apotex Inc, 2014 FC 638 [AstraZeneca FC]), in which Justice Rennie acknowledged that the meaning of the “inventive concept”
of a patent’s claims was the subject of controversy, and that the parties before him, as they do before me, adopted conflicting interpretations of the inventive concept. He noted that “the parties had conflicting views on the legal principles underpinning the inventive concept as well. AstraZeneca, in its closing, argued that the inventive concept, promise of the patent, and claims construction, are ‘just one construction for all purposes.’ By contrast, Apotex argued that all three exercises are distinct inquiries. Such a stark contrast in the basic legal framework underlying key doctrines in patent law, between two highly sophisticated litigants, is alarming to say the least”
(at para 266). Justice Rennie determined that, similar to claim construction, the identification of the inventive concept begins with the claims, and the remainder of the patent may be consulted only if necessary (at para 266). Justice Rennie, ultimately, found there was no need to look to the disclosure for improved properties within the inventive concept of the 653 patent because a viable inventive concept was present in the claims alone. The FCA confirmed the FC directed itself to the correct legal test and the SCC did not address the issue.
[287]
Justice Rennie’s interpretation of Sanofi’s inventive concept, like that of the FCA decision, centered on the patent’s claims, and the SCC did not displace this interpretation.
(vi)
The meaning of the term inventive concept
[288]
The element that must be compared with the prior art in the obviousness analysis has been named “the solution taught by the patent”
in Beloit, the “inventive concept” in Sanofi, and is named the “subject-matter defined by a claim” in the Patent Act.
[289]
It appears clear from the afore-mentioned decisions of the FCA that these terms all mean the same thing and that they relate to the essential elements of the claims, identified by the claim construction.
[290]
The Courts have acknowledged that recourse to elements of the disclosure may be permitted when there is ambiguity in the claims or when the inventive concept is not discernable from the claims. For example, in the context of a selection patent, the inventiveness has been found to lie in the advantages the selection presented over the genus (Astrazeneca SCC at para 31; Sanofi at para 77; Novopharm FCA). The FCA and the Court confirmed that a distinction must be made between the invention and what are alternatively called properties of the invention, benefits of the invention, or results of the invention (BMS FCA at para 74; Apotex v Pfizer 2019 FCA 16 para 37–45; Hospira FCA at para 94; the 684 Patent NOC decision at para 164). There is no need to look to the disclosure for improved properties, if a viable inventive concept is present in the claims alone (AstraZeneca FC at para 272).
[291]
Focusing on the essential elements of the claims when conducting the obviousness enquiry accords with overarching principles of patent law. As the Defendants point out, the statute refers to the subject-matter defined by a claim, not by a patent. The patent law is wholly statutory, and the statute itself directs us to focus on the claims.
[292]
Sections 28.2 and 28.3 of the Patent Act both refer to the subject-matter defined by a claim to identify the element that must be assessed against the prior art. As per the general rules of interpretation, the same term in both sections should mean the same thing, and the same element should thus be used as the point of comparison against the appropriate prior art in both the anticipation and the obviousness enquiries. “Giving the same words the same meaning throughout a statute is a basic principle of statutory interpretation (Elmer Driedger, Construction of Statutes (2nd ed. 1983), at p. 93)”
(R v Zeolkowski 1989 1 SCR 1378; Ruth Sullivan, Sullivan on the Construction of statutes (Markham: LexisNexis, 2014) at §8.34; Aux Sable Liquid Products LP v JL Energy Transportation Inc 2019 FC 581; Zero Spill FCA).
[293]
The subject-matter defined by a claim of the anticipation analysis resides in the essential elements of the claims. Hence, given the general rules of interpretation, the subject-matter defined by a claim of the obviousness analysis should reside in the same essential elements.
[294]
Finally, the SCC taught us that claim construction is antecedent to the validity and infringement analyses, that it serves all purposes, and that the key to purposive construction is the identification of the essential elements of the claims. As the infringement analysis is concerned with the essential elements of the claims, the validity analysis, which include the obviousness analysis, should also be concerned with the essential elements of the claims.
(vii)
The subject-matter defined by a claim of the asserted Claims of the 540 Patent
[295]
The results of the PSR, ie the CIAT, equilibration, reaction speed, high purity, or high yield, are not mentioned in the claims of the 540 Patent, the parties have not asked the Court to construe them in the claims, and they have not been. They are thus unclaimed benefits, or results, of the invention. Based on the FCA’s recent decisions, I must not accept them as the subject-matter defined by a claim if a viable subject-matter is found in the claims. It appears the 540 Patent claims particular means to achieve the results, not the desirable results themselves (see Free World Trust at para 32; BMS FCA at para 74; Hospira FCA at para 94).
[296]
In any event, a subject-matter defined by a claim is here discernable from the essential elements of the claims as construed. It is, for each of the asserted Claims 1, 3–4, 7–10 and 12 a method of preparing a compound comprising a sequence of steps. I accept Dr. Anderson’s description at para 220 of his Expert Validity Report: “In my opinion, the inventive concept of the claims of the 540 Patent, is a method for the formation of the compound tadalafil, and related compounds that relies on a Pictet‐Spengler reaction where in the desired cis‐diastereomer of the tetrahydro‐β‐carboline product is insoluble in the reaction solvent and the undesired trans‐diastereomer is soluble at reflux temperature or below. For Claim 12, in particular, the inventive concept would include the above method wherein the reaction solvent is iso‐propanol.”
[297]
The obviousness inquiry should be undertaken on a claim-by-claim basis (Zero Spill FCA at para 85). If an independent claim is found not to be obvious, then cascading, narrower dependent claims therefrom cannot be obvious. In contrast, if an independent claim is held to be obvious, the Court must go on to consider each of the cascading, narrower dependent claim for obviousness.
(e)
Third step: Identify what, if any, differences exist between the matter cited as forming part of the “state of the art”
and the inventive concept of the claim or the claim as construed;
[298]
Apotex relies on two prior art publications, the 594 Application and the 377 Patent (equivalent to the US 006 Patent), and Lilly agree that these are part of the prior art. I have outlined each earlier. Lilly assert that the 008 Patent is another piece of prior art.
(i)
Claims 1, 3–4
[299]
For Claim 1a and Claim 3, regarding the esterification step with methyl alcohol (methanol) to make the D-tryptophan methyl ester hydrochloride salt from D-tryptophan, Apotex submits that it was already taught by the prior art, and Dr. Laird already conceded on the matter (transcript of January 16, 2020 at page 127). It appears, based on the evidence, that this a very commonly used and well-known reaction. There is therefore no difference between the prior art and the subject-matter defined by the claim.
[300]
For Claim 1b and Claim 4, with respect to the PSR, Apotex asserts that it was taught by Intermediate 1 of Example 2 in the 594 Application, and further taught by the epimerization (interconversion of trans into cis or a mixture thereof) reactions of Intermediate 69 at page 26 in the 377 Patent. Apotex also argues that the 377 Patent teaches the PSR with toluene and benzene, which are two solvents said to be useful for the PSR in the disclosure of the 540 Patent. For this step, Apotex asserts that there is no difference between the state of the art and the subject-matter defined by the claim.
[301]
Lilly insist that there are differences and denies that the PSR in Claim 1b and Claim 4 is taught by the 594 Application because the nature of the resulting suspension in the 594 Application is not disclosed, thus arguing that nothing would suggest that the cis reaction product to be insoluble and the trans soluble. Additionally, Lilly argue that the crystallization occurs after cooling and the addition of ethyl acetate and MTBE, and the temperature at which the reaction is run is at 50 C instead of being at reflux temperature of that solvent at 118 C (the 540 Patent discloses PSR using isopropyl alcohol run between 70 C and 82 C, close to the reflux temperature of that solvent). Against the 377 Patent, Lilly also argue, citing Dr. Anderson (transcript of January 21, 2020 at page 54), that the 377 Patent teaches a process by which the cis-diastereomer remained in solution during the PSR despite low temperature, instead of crystallizing out. After the completion of the PSR, only subsequent processing, Lilly insist by citing Dr. Laird, led to the crystallization of the trans-isomer rather than the cis (transcript of January 16, 2020 at page 113). Despite the conversion of the trans to the cis in subsequent processes, the cis did not crystallize out according to Lilly because the yellow solution still required processing (transcript of February 6, 2020 at page 154), which Apotex does not agree with. Lilly note that, citing Dr. Laird, if you compare the productivity of the 594 process and the 540 process on a similar scale, as well as the reaction time, the 540 process has a much better yield and is much faster.
[302]
Given that I have accepted the evidence concluding that the suspension is the desired cis-diastereomer, I find no difference between the prior art and Claim 1b and Claim 4.
[303]
For Claim 1c, regarding the step of separating from the mixture, I find that it was disclosed by the process in Intermediate 1 Example 2 of the 594 Application, and also by the filtration process of the 377 Patent.
[304]
As there is no difference between the subject-matter defined by the essential elements of Claims 1, 3–4 and the prior art, Claims 1, 3 and 4 are obvious and therefore invalid.
(ii)
Claims 7–10
[305]
Claim 7a is simply to provide a desired diastereomer of a tetrahydro-β-carboline by the method of Claim 1. Given my conclusion on Claim 1, I find no difference between Claim 7a and the prior art.
[306]
For Claim 7b, regarding the acylation of the tetrahydro-β-carboline, Apotex submits that it was already taught by the prior art, and Dr. Laird already conceded on the matter (transcript of January 16, 2020 at page 127). Pathway of the 377 Patent discloses a process that is akin. There is thus no difference between Claim7b and the prior art.
[307]
For Claim 7c, and Claims 8–10, regarding the amination cyclization of the chloroacetyl carboline (transcript of January 16, 2020 at page 127), Apotex submits that it was already taught by the prior art, and Dr. Laird already conceded on the matter (transcript of January 16, 2020 at page 127). Pathway of the 377 Patent again discloses a process that is akin. There is thus no difference between Claim 7c and the prior art.
[308]
For Claim 7d, regarding the purification with glacial acetic acid, Apotex acknowledge that this constitute a difference between the prior art and subject-matter of the claim (Apotex Closing Memorandum at page 78).
[309]
I thus find there is a difference between Claim 7d and the prior art. The same difference subsists between Claims 8–10 and the prior art.
(iii)
Claim 12
[310]
Claim 12a is similar to Claim 1a, Claim 12c is similar to Claim 7b, and Claim 12d is similar to Claim 7c. They are disclosed in the prior art, and there is no difference between the prior art and the subject-matter defined by the claims. The validity of Claim 12 thus depends upon Claim 12b, the PSR in isopropyl alcohol at reflux temperature.
[311]
The PSR with isopropyl alcohol as a solvent with D-tryptophan methyl ester hydrochloride and piperonal as reactants of Claim 12b is not found in the prior art. There is thus a difference between the subject-matter as defined by Claim 12b and the prior art.
[312]
As I have found a difference between the subject-matter of Claim 7d and Claim 12b and the prior art, I must examine if these differences constitute steps that would have been obvious to the PSA or if they require a degree of invention.
(f)
Fourth step: Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?
(i)
Introduction
[313]
As demonstrated by the invention story detailed by Mr. Pawlak and Dr. Martinelli, this is an area of science where experimentation is common. In fact, Apotex alleges that, in both cases, routine solvent screening would have led to the invention. Dr. Martinelli outlined how typical, simple, and straightforward screening solvents is, and that it would be done in a small cup sized vessel, instead of a production vessel of the size of a room (transcript of January 14, 2020 at pages 75–76). Mr. Pawlak, himself, performed a number of solvent screens, and also experimented with different acids and reaction conditions, both to develop processes, but also to make adjustments and optimize them. Determining obviousness in this context thus warrants the application of the obvious to try test.
(ii)
Obvious to try
[314]
In Sanofi, the Court introduced at the fourth step the “obvious to try”
test, where a court may consider whether the claimed invention was “obvious to try”
. Not every case will require an application of the “obvious to try”
test; it may be appropriate in instances where the art in question encompasses advances made as a result of experimentation (Sanofi at para 68).
[315]
The SCC in Sanofi provided a non-exhaustive list of factors to consider in determining whether the invention was “obvious to try”
(Sanofi at paras 69–70):
[69] If an “obvious to try” test is warranted, the following factors should be taken into consideration at the fourth step of the obviousness inquiry. As with anticipation, this list is not exhaustive. The factors will apply in accordance with the evidence in each case.
1. Is it more or less self-evident that what is being tried ought to work? Are there a finite number of identified predictable solutions known to persons skilled in the art?
2. What is the extent, nature and amount of effort required to achieve the invention? Are routine trials carried out or is the experimentation prolonged and arduous, such that the trials would not be considered routine?
3. Is there a motive provided in the prior art to find the solution the patent addresses?
[70] Another important factor may arise from considering the actual course of conduct which culminated in the making of the invention. It is true that obviousness is largely concerned with how a skilled worker would have acted in the light of the prior art. But this is no reason to exclude evidence of the history of the invention, particularly where the knowledge of those involved in finding the invention is no lower than what would be expected of the skilled person.
[316]
The “obvious to try”
test is not meant to replace all previous inquiries, and other inquiries remain possible (BMS FCA at para 60). As outlined in the recent decision of Apotex Inc v Pfizer Canada Inc, 2019 FCA 16 at para 32, the “obvious to try”
test must be approached with caution:
[32] Following Sanofi, our Court in Atazanavir echoed the Supreme Court’s consideration of obviousness by reiterating that the “obvious to try” test must be approached with caution as it remains one factor amongst many that may assist in the obviousness inquiry (Atazanavir at para. 38; Sanofi at paras. 64-65). Our Court in Atazanavir explained that the “obvious to try” test introduced by Sanofi had in no way displaced other tests, including the test set out in Beloit. Our Court expressly recalled that while the Supreme Court introduced the “obvious to try” test, it favours “an expansive and flexible approach that would include ‘any secondary considerations that [will] prove instructive’” (Atazanavir at para. 61, referring to Sanofi at para. 63). As a result, a categorical approach to the obviousness inquiry and the elaboration of a “hard and fast rule” was specifically deemed inappropriate and rejected by our Court (Atazanavir at para. 62).
[317]
When an expert is hired for the purpose of testifying, a court must be wary of his or her hindsight bias (Bridgeview Manufacturing Inc v 931409 Alberta Ltd (Central Alberta Hay Centre), 2010 FCA 188 at para 50 [Bridgeview]). It is not fair for a person claiming to have invented a combination invention to break the combination down into its parts and find that, because each part is well known, the combination is therefore obvious (Bridgeview at para 51).
[318]
The risks of hindsight bias are aptly stated by Justice Sharlow in Apotex Inc v Bayer AG, 2007 FCA 243 at paras 25-26:
[25]…The traditional warning about hindsight is found in Beloit (at page 295, per Hugessen J.A.):
Every invention is obvious after it has been made, and to no one more so than an expert in the field. Where the expert has been hired for the purpose of testifying, his infallible hindsight is even more suspect. It is so easy, once the teaching of a patent is known, to say, "I could have done that"; before the assertion can be given any weight, one must have a satisfactory answer to the question, "Why didn't you?"
[26] This does not mean that the trier of fact is required as a matter of law to reject an expert’s hindsight analysis. After all, the evidence of a party alleging invalidity for obviousness is necessarily based to some degree on hindsight because it is addressed to a hypothetical question about a point of time in the past. However, as a factual matter, an allegation of obviousness may be weakened if the evidence does not explain, directly or by inference, why the Claimed invention was not discovered by others.
Is it more or less self-evident that what is being tried ought to work? Are there a finite number of identified predictable solutions known to persons skilled in the art?
Claim 7 step d and Claims 8–10
[319]
Dr. Anderson indicated that routine solvent screening would allow a skilled person to uncover glacial acetic acid as a recrystallization and purification solvent. He notes that the textbooks available to the PSA before July 31, 2002 listed glacial acetic acid as a potential recrystallization solvent, and opines that the PSA would therefore not have required inventive ingenuity to bride the gap and the application of glacial acetic acid in the recrystallization of tadalafil and structurally related compounds (Dr. Anderson Expert Report at paras 242–243).
[320]
I note this assertion of lack of inventiveness is unopposed by Lilly, and I retain Dr. Anderson’s opinion as carrying the most weight to establish that it was more or less self evident that what was being tried ought to work. It is a simple solubility difference between the desired compound, and the common impurities at a given temperature. No reaction needs to be run.
Claim 12 step b
[321]
Apotex takes the position that a routine solvent screening would have revealed that isopropyl alcohol, which is a commonly used solvent, would work for the PSR in Claim 12b. According to Apotex, isopropyl alcohol was obvious to try in a routine solvent screening. It argues that the 540 Patent admits that the selection of a particular solvent, in this case, the isopropyl alcohol, is well within the ability of the skilled person as reiterated twice in the 540 Patent disclosure, which renders Claim 12 obvious in light of the 594 Application and 377 Patent.
[322]
In the event that differential solubility and equilibration is brought into Claim 12, Apotex argues that the re-epimerization reaction disclosed under Intermediate 69 of the 377 Patent teaches that the trans-diastereomer can be converted into cis in the presence of certain acids and heat, with the cis directly crystallizing out. Apotex submits the PSA ought to try to screen various solvents, and test the solubility of the compounds involved in the PSR in various solvents such that only the desired diastereomer crystallizes out directly from the reaction.
[323]
Apotex points out that method B and method C of Intermediate 69 in the 377 Patent disclose processes in which the desired cis-diastereomer directly crystallizes, unlike what Lilly describe.
[324]
Lilly argue that the 377 Patent taught away from the 540 Patent by disclosing, under Intermediates 67 and 68 of the 377 Patent, that the trans crystallized first. The methods in A, B, and C of Intermediate 69 of the 377 Patent also do not involve crystallization, according to Lilly. The processes in Intermediates 54 and 55, 67 and 68 are slow and produce a mixture of cis and trans.
[325]
Lilly also argue that the 008 Application, which discloses a reaction with an analogue of tadalafil, also teaches away from the 540 Patent because, despite the use of isopropyl alcohol, the yield is poor and there is no crystallization. A PSA would learn from the failures, including what is taught by the 008 Patent.
[326]
According to Lilly, consequently, the inventors took a leap into the dark when they developed the novel and non-obvious process of using a CIAT in isopropanol, despite the prior art (the 008 Application) teaching away from the use of this solvent. Lilly also add that the process in the 540 Patent is highly commercially valuable being fifteen times more productive than the 594 process.
[327]
Lilly also add that Dr. Williams indicated that the |||| reports he reviewed contained the precise type of |||||||||||||||||||| the skilled person would undertake (Dr. Williams Expert Report at para 230), yet |||| never found the solution.
[328]
Finally, Lilly argue that the test is not whether something is “worth a try”
but whether it is “obvious to try”
, and warns against hindsight. “Worth to try”
was specifically rejected by the SCC in Sanofi at paras 55, 60, 68. Lilly argue that the mere possibility that something might turn up is not enough (Eli Lilly Canada Inc v Mylan Pharmaceuticals ULC, 2015 FCA 286 at para 4). Lilly argue that trying the concomitant crystallization in reaction of the desired cis-diastereomer and using isopropyl alcohol as a solvent in a PSR were not self-evident.
[329]
The evidence shows it is self-evident that there is a finite number of solvents that the PSA ought to have tried (as well as other potential solvents the PSA could have tried), and that isopropyl alcohol is one of the solvents that PSA ought to have tried. It would be clear for the PSA that a number of solvents could have been used. Upon testing, the PSA would find that isopropyl alcohol is one of those that can be used for the PSR. Dr. Anderson explained that there is a finite number of reaction solvents that are suitable to use in a commercial scale (Dr. Anderson Expert Validity Report at para 250).
[330]
It is important to note, again, that Claim 12b, as it is written, claims isopropyl alcohol as a solvent but does not claim CIAT, equilibration, yield, or purity, and that these effects have not been construed in the claims nor considered as the subject-matter defined by a claim.
[331]
Dr. Anderson has presented a list of commonly used solvents that includes isopropyl alcohol (Dr. Anderson Expert Validity Report at para 235). The PSA, trying to find a better solvent than dichloromethane in the 377 Patent and acetic acid in the 594 Application, would eliminate a number of options on that list (or would try them with under fewer reaction conditions), especially those that are low cost, low toxicity, environmentally friendly, and easily commercially accessible. Isopropyl alcohol is one of such solvents, despite the poor yield disclosed in the 008 Patent because, as Dr. Williams opined, a hydroxyl group on the tryptophan ring as in the molecule exemplified in the 008 Patent could substantially affect bonding behaviors of the tryptophan (transcript of January 24, 2020 at page 70). Although the PSA would try to run the reaction at different temperatures, the PSA would attempt to run it as hot as possible for higher reaction speed. It would not be uncommon for the PSA to attempt to run the reaction at reflux temperature, or only slightly below reflux. The solution with isopropyl alcohol would have been found by the PSA with routine solvent screening, and the PSA would have achieved a decent yield by selecting typical reaction conditions.
What is the extent, nature and amount of effort required to achieve the invention? Are routine trials carried out or is the experimentation prolonged and arduous, such that the trials would not be considered routine?
[332]
Apotex argues that a solvent screen is routine just as Dr. Martinelli carried it out. It adds that the inventors of the 540 Patent reached the invention after only |||||||||||||||||||||| conducted in ||||||||||||||||||||||||, and that Dr. Martinelli characterized solvent screening as “pretty typical”
, “pretty simple”
and “fairly straightforward”
(transcript of January 14, 2020 at pages 75–76). Apotex also refers to Dr. Anderson Expert Validity Report at paras 263–264 in which Dr. Anderson indicated that he found no evidence from Mr. Pawlak’s notebook that there was anything arduous in identifying a better process.
[333]
Mr. Pawlak in his testimony indicated that up to ten PSRs could have been run in parallel if he wished (transcript of January 15, 2020 at page 59).
[334]
Lilly recognize that routine trials are generally carried to find better processes (Lilly Closing Memorandum at para 368). |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||| ||||||||||||||||||||||||||||||||||||||||||, without success: the desired diastereomer did not crystallize out, the yield was poor and there were a number of impurities. ||||||||||||||||||, and |||| did not accidently find what Mr. Pawlak and Dr. Martinelli have found over |||||||||||| of work. Lilly challenge Dr. Anderson’s expert deposition that he found nothing arduous or inventive with the process because Dr. Anderson did not receive |||| reports. Lilly add that the best |||||||| and |||||| could come up for the process prior to Lilly getting involved was the |||||||||||||||||||||||||||||| process which was slow and which only had a yield between ||||||||, with also a high number of impurities.
[335]
As per Dr. Martinelli and Mr. Pawlak’s testimony, the choice of solvent is routine and the entries in Mr. Pawlak’s notebook indicate it did not take long nor a high number of attempts to reach the solution. As per Dr. Laird’s opinion, a poor yielding process with impurities may be acceptable for initial preclinical and clinical tests. Despite full months of work by ||||||, only || ||||||||||||||||||||||||||||||||||||||||||||||||||, have been used to screen various solvents before concentrating on the |||||||||||||| solvent starting in |||||||||||||| (Dr. Laird Validity Report at para 141). |||||||||||| also did not appear to have intensely screened a large number of solvents. They tried |||||||||| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| |||||||||||||||||||||| |||||||||||||||||||||||||||||||||||||||||||| as in the previous process from |||||||||||||||| as well as ||||||||||||||. This is not a substantial number considering that these solvents screens can be run in parallel, ten solvents and reaction conditions at the time. Moreover, |||||||| is toxic, and |||| ought to know that it is an unsuitable candidate for large commercial processes.
[336]
Dr. Anderson’s opinion that the work is routine was convincing despite the fact that he did not see the |||| report as ||||’s focus was finding small sized processes (larger than medicinal chemistry processes) to complete the clinical trials. Dr. Martinelli and Mr. Pawlak confirmed that solvent screening was routine and it took them about |||||||||| and what appears to be a |||||||||||||||| of experiments to find the proper solvent.
[337]
I am cognizant of the danger of hindsight; however, the evidence shows that the work done by Dr. Martinelli and Mr. Pawlak on the PSR was not long and arduous.
Is there a motive provided in the prior art to find the solution the patent addresses?
[338]
Apotex argues that the 594 Application and the 377 Patent re-epimerization examples provides enough motive to analyze the solubility of the compounds involved in the PSR in various solvents. It also points to Dr. Anderson’s opinion that the skilled person would undoubtedly be motivated to adapt and improve existing synthetic processes taught in the prior art to prepare tadalafil and related compounds. Dr. Anderson continued and explained that in light of this motivation, the PSA would try, through regular screening experiments, different reagents and solvent choices (Dr. Anderson Expert Validity Report at para 249). As acetic acid is a polar protic solvent, the PSA would have the motivation to pursue and to test other polar protic solvents (Dr. Anderson Expert Validy Report at para 251).
[339]
Lilly respond that the SCC held in Sanofi at para 90 that the motive must be to specifically pursue the invention of the patent at issue. The motive must point directly to the solution in the 540 Patent. They argue that Dr. Laird indicated that nothing in the prior art pointed towards the solution taught by the 540 Patent, and that there was no direction on how to get around the disadvantages in the prior art (Dr. Laird Validity Report at para 134; transcript of January 16, 2020 at page 119).
(iii)
Fourth step conclusion
[340]
As mentioned earlier, the subject-matter defined by a claim do not encompass the results of the PSR, ie the CIAT, high yield, high purity, speed as they are not essential elements of the claims, which provided a viable subject-matter for obviousness inquiry.
[341]
While I recognize that the factors suggested in Sanofi are not exhaustive, I am satisfied upon review of the above factors that the difference between the prior art and the subject-matter of Claims 7 and 12 are not inventive.
(3)
Conclusion on obviousness
[342]
Apotex’s allegations of obviousness are demonstrated and all asserted Claims are invalid for obviousness.
D.
Inutility/Inoperability
[343]
Apotex argues that if the Court fails to correct the obvious mistake in Claim 12, the claim would be invalid on the basis of inutility.
[344]
Lilly assert that Claim 12 is clearly useful, as the mistake does not invalidate the claim. In fact, Lilly is not seeking to have the Court rewrite the elements of Claim 12 as it relates to the misnaming of the compound provided in Claim 12c, but simply to accept the evidence of the experts providing how a PSA would read the claim. An invention must be new and useful (Patent Act, s 2). For it to be useful, there must be a scintilla of utility, and the promise doctrine needs not be brought back (AstraZeneca SCC).
[345]
Citing Sanofi, Lilly argue that just as “obvious errors or omissions in the prior patent will not prevent enablement if reasonable skill and knowledge in the art could readily correct the error or find what was omitted”
, the error would not render the claim inoperable.
[346]
Apotex asserts, however, that Lilly should have asked the Patent Office for a correction at a much earlier stage because Parliament has put into place a specific timeline for the corrections in the Patent Act, notably section 47, and in the Patent Rules, SOR/2019-251, notably section 109. Apotex mentions that the US equivalent of the 540 Patent was corrected prior to the issuance of the 540 Patent.
[348]
Dr. Anderson wrote in his Expert Validity Report at para 267 that “if the skilled person literally followed the method of Claim 12, it would not produce the target compound tadalafil”
, but “if the skilled person followed the sequence of actions described in Claim 12 without regard to the product of each of the steps, then the target compound, tadalafil, would be produced”
.
[349]
Based on Dr. Anderson’s opinion, I am satisfied that the PSA would understand Claim 12 to bear a mistake, and would make tadalafil by following the sequence of actions. Apotex has not demonstrated there is an issue in understanding the claim. Therefore, Apotex has not met its burden for its allegation of inutility.
E.
Overbreadth
[350]
Apotex alleges that Claims 1, 3, 4 and 7–10 extend beyond what was actually invented because Dr. Doecke, another named inventor of the 540 Patent, under the supervision of Dr. Martinelli, investigated |||||||||||||||| as solvent, but the desired cis-diastereomer did not crystallize in solution (transcript of January 14, 2020 at pages 97–98). Nevertheless, Apotex asserts that Lilly falsely contend in page 22 of the 540 Patent that |||||||||||||||| would work by having it on the second list of useful solvents. The heart of Apotex’s argument is thus whether |||||||||||||||| is within the scope of Claim 1b or outside of the scope of Claim 1b.
[351]
Lilly submit, first, looking purely at the law, that it is clear they have not set the fences broader that the invention made; and second factually, that Apotex has not met its burden in terms of the evidence that they have attempted to put forward to support its allegation. They assert that different solvents can be used and they must respond to the limitation, hence solvents in which the desired diastereomer crystallises. If a solvent does not meet that imitation, it is not claimed.
[352]
Lilly urge the Court to avoid applying the doctrine of overbreadth, which Lilly argue is a non-statutory invalidity allegation. It further argue that overbreadth has the potential to become the new promise of the patent, an attack on the claims made by parsing the disclosure. Furthermore, Lilly argue that Apotex has put forward no evidence to support the overbreadth attack since only Dr. Williams opined on overbreadth, but no legal instructions on overbreadth was joined to his report.
[353]
The doctrine of overbreadth is what its name suggests. It exists to ensure that the grant of a monopoly is limited to the invention made and disclosed in the specification (Farbwerke Hoechst AG v Commissioner of Patents, [1966] Ex Cr 91 at 106):
There are two fundamental limitations on the extent of the monopoly which an inventor may validly claim. One is that it must not exceed the invention which he has made, the other is that it must not exceed the invention he has described in his specification.
[354]
The reasoning is that exclusivity must not be granted overbroadly (RCA v Raytheon Manufacturing Co, [1956-60] Ex Cr 98). A reasonable delineation of the scope of the patent is necessary (Free World Trust at para 42).
[355]
As such, overbroad claims are invalid (Amfac Foods Inc v Irving Pulp & Paper (1986), 13-12 CPR (3d) 193 (FCA) [Amfac]). To overclaim “is to lose everything”
(Biovail Pharmaceuticals v Canada (Ministry of National Health and Welfare), 2005 FC 9 at para 8). A claim would be overbroad if essential elements are omitted (Amfac).
[356]
The way that Apotex articulated the allegation of overbreadth in this case appears indeed very akin to the promise doctrine, abolished in AstraZeneca SCC. What Apotex really asks the Court to do is to parse the disclosure, conclude that |||||||||||||||| promises to be useful for the PSR, import |||||||||||||||| into Claim 1 in the absence of any ambiguity, and strike Claim 1 as a result.
[357]
The SCC warned against this doing this (AstraZeneca SCC at para 31):
The Promise Doctrine, by contrast, directs courts to read both the claims and the disclosure to identify potential promises, rather than the claims alone, even in an absence of ambiguity in the claims. After a process of identifying promises, the doctrine equates the fulfillment of these promises (by demonstration or sound prediction) with the requirement in s. 2 that an invention be useful. The doctrine then goes on to provide that if any one of the promises is not fulfilled, then the utility requirement in s. 2 is not met and the patent, in its entirety, is invalid.
[358]
As such, the doctrine of overbreadth should not be applied in the manner suggested by Apotex, akin to the promise doctrine (see Les Laboratoires Servier v Apotex Inc, 2019 FC 616 at para 237; Apotex Inc v Abbott Laboratories, Limited, 2018 ONSC 5199 at paras 8, 27–28).
[359]
Apotex has not met its burden to establish that Claims 1, 3-4, 7-10 are invalid on the ground of overbreadth.
F.
Conclusion on invalidity
[360]
As per the reasons above, I find Claims 1, 3–4 are invalid for anticipation and all of the asserted Claims are invalid for obviousness.
VII.
Lilly’s claim of infringement
[361]
Having concluded that all the claims are invalid, I would not need to examine infringement. However, in case I am wrong on the validity issue, I will examine Lilly’s allegations.
A.
Principles
[362]
Under section 42 of the Patent Act, the patentee and their legal representatives have the exclusive right, privilege and liberty of making, constructing, and using the invention and selling it to others to be used, and, under section 44, the term limited for the duration of the patent is twenty years from the filing date. Subsection 55(1) provides that a person who infringes a patent is liable for all damages sustained by the patentee by reason of the infringement.
[363]
As stated earlier, and since neither the statutory presumption at section 55.1, nor the common law (Hoffmann) presumption applies to reverse the burden, it falls, here, on Lilly to prove the Apotex processes infringe the patent’s asserted Claims (Monsanto Canada Inc v Schmeiser, 2004 SCC 34 at para 29). Apotex has four processes: ||||||||||, ||||||||||||||||||||||, |||| |||||||||||||||||||||||| and ||||||||||||||||||||||||.
[364]
To determine whether any claim of a patent is infringed, a court must purposively construe the claims of the patent, which I have done, and then determine whether the allegedly infringing product falls within the scope of those claims (Free World Trust, above at paras 48-49). There is no infringement if an essential element is different or omitted. There may still be infringement, however, if non-essential elements are substituted or omitted. (Free World Trust at para 31).
[365]
Once the construction is completed and the essential and non-essential elements are identified, the Court must then determine whether any of the processes used by Apotex contain all of the essential elements of the asserted Claim as construed. Patent infringement requires that the Defendant has misappropriated all of the essential elements of a valid patent claim (Free World Trust paras 68 and 75). If even one essential element is omitted from the Defendant’s alleged infringing activities, there is no infringement (Zero Spill FCA at para 56).
[366]
Mr. Bagga confirmed, in his testimony, that Apotex’s approvals from the Minister of Health to use each of these four processes are current.
[367]
The infringement allegations regarding the |||||||||| and the |||||||||||||||||||||||| processes turn on the claims construction. The allegations regarding the |||||||||||||||||||||||||||||||||| process turn on the regulatory exemption, while the allegations on the |||||||||||||||||||||| turn on the evidentiary burden.
B.
Apotex process: ||||||||||
[368]
In regards to the independent Claim 1, Apotex does not dispute that the |||||||||| process includes all its essential elements. Neither Dr. Anderson nor Dr. Williams provided an opinion with respect to infringement of Claims 1, 3-4 of the 540 Patent by ||||||||||, while Dr. Laird opines it does infringe Claim 1, 3-4.
[369]
I note that the |||||||||| process contains a step of |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| ||||||||, ie a step of ||||||||||, in addition to the elements Dr. Laird analyses based on his claim construction (Apotex Infringement Expert report of Dr. Trevor Laid pages 8-9) which is the essential element of Claim1c as I have construed it. I also note that the |||||||||| process uses |||||||||| and ||||||||||||||||||. I am satisfied that Lilly has met their burden and established that the |||||||||| process infringes Claims 1, 3-4 of the 540 Patent, should those claims be valid.
[370]
In regards to Claim 7, Dr. Anderson notes that the |||||||||| process does not encompass the step of |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| of Claim 7d (Dr. Anderson Responding Report at para 74).
[371]
I have construed the purification step of Claim 7d as an essential element, hence the fact that it is omitted takes the |||||||||| process out of the scope of the monopoly. The |||||||||| process does not infringe Claim 7, nor Claims 8-10 as they encompass the method of Claim 7.
[372]
In regards to Claim 12, Dr. Anderson notes that the |||||||||| process does not use isopropyl alcohol as a solvent for the purposes of Claim 12b, but uses |||||||||| (Dr. Anderson Responding Report at para 78).
[373]
I have construed the use of isopropyl alcohol of Claim 12b as an essential element, and the fact that it is omitted from the |||||||||| process again takes it outside the scope of the monopoly. I find that the |||||||||| process does not infringe Claim 12.
C.
Apotex process: ||||||||||||||||||||||||
[374]
In regards to Claim 1, both Dr. Laird and Dr. Anderson, respectively Lilly’s and Apotex’s expert, agree that the |||||||||||||||||||||||| process does not contain a |||||||||||||||||||||||||| step of the ||||||||||||||||||||||||||||||||||||||||||||||||||. The reaction mixture, after the completion of the PSR, continues with the ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||.
[375]
I have construed Claim 1c as a step of physically separating the cis-diastereomer from the mixture and as an essential element. The fact that an essential element is omitted takes the process outside of the scope of the monopoly, and the |||||||||||||||||||||||| process therefore does not infringe Claim 1, nor Claims 3-4 as they encompass the method of Claim 1.
[376]
In regards to Claim 7, Dr. Anderson opines that the process does not encompass Claim 7a, as the |||||||||||||||||| is not provided through the process of Claim 1, nor Claim 7d, as the |||||||||||||||||||||||||||||||||||||||||| step |||||||||||||||||||| is not performed. Dr. Laird construed Claim 7d as a non-essential element.
[377]
I construed the step of purifying of Claim 7d as an essential element, and the fact that it is omitted, in addition to the fact that the |||||||||||||||| is not provided through the process of Claim 1, takes the |||||||||||||||||||||||| process outside the scope of the monopoly. The |||||||||||||||||||||||| process does not infringe Claim 7, nor Claims 8-10, which encompass the method of Claim 7.
[378]
In regards to Claim 12, Dr. Anderson also opines that the |||||||||||||||||||||||| process does not infringe upon Claim 12 because the PSR is not performed with isopropyl alcohol, but rather ||||||||||||||. Dr. Laird’s analysis is premised on the addition of equivalents to certain elements of the claim.
[379]
I have construed isopropyl alcohol to be an essential element of Claim 12 and the fact that it is omitted from the |||||||||||||||||||||||| process thus takes it outside the scope of the monopoly. The |||||||||||||||||||||||| process does not infringe Claim 12.
[380]
In conclusion, the |||||||||||||||||||||||| process infringes none of the asserted Claims.
D.
Apotex process: ||||||||||||||||||||||||||||||||
[381]
First, it appears clear from Prothonotary Tabib’s bifurcation Order that infringement issue are to be determined at this stage while the extent or quantification are to be decided at the second stage.
[382]
Section 55.2 of the Patent Act states that “it is not an infringement of a patent for any person to make, construct, use or sell the patented invention solely for uses reasonably related to the development and submission of information required under any law of Canada, a province or a country other than Canada that regulates the manufacture, construction, use or sale of any product”
. As per the wording of the statute, regulatory use, should it be established, is not infringement and, despite the parties’ Joint Statement of Issues, whether it applies or not must be decided here in the liability phase.
[383]
Lilly argue that Apotex bears the burden of claiming an exemption under section 55.2 of the Patent Act (Teva Canada Limited v Novartis AG, 2013 FC 141 [Norvatis]), and that it has not met its burden. Lilly submit that Apotex has not provided any evidence at all as to the ultimate destination of the material said to have been developed for regulatory purposes. Lilly add that Apotex has not led evidence to show what as been imported was for regulatory purposes, nor that none remains, that they cannot do it in the future or that it cannot be formulated or sold and finally, that what remained from those lots has been destroyed. They rely on Apotex Inc v Sanofi-Aventis, 2011 FC 1486 at paras 232–238, rev’d on other grounds 2013 FCA 186, and on Novartis that confirmed this evidence was necessary. As per Lilly’s submissions, Mr. Bagga’s testimony that none of the material has been used for anything but regulatory purposes is insufficient.
[384]
Apotex does not contest that the essential elements of all claims are found in the process, but raises the regulatory exemption under 55.2 of the Patent Act. They point to Mr. Bagga’s testimony, that the || material was only used for regulatory testing, that it was not converted into finished dose, that the material was not tableted, that it was not sold in the Canadian market, that it was not formulated nor sold for export. Apotex cites Justice Hughes in Merck & Co Inc v Apotex Inc, 2006 FC 524 at para 153, var’d on other grounds 2006 FCA 323 for the proposition that the exception is clear and non equivocal, as well as Justice Gauthier in Cefaclor for the proposition that the exemption is not limited to material actually provided to a regulator.
[385]
I note that the only evidence comes from Mr. Bagga, is uncontradicted, and confirmed all the material was for regulatory purposes. Based on the evidence, it appears more probable than not that this is the case, that the exemption of section 55.2 applies and that the |||||||||||| |||||||||||||| process therefore does not infringe.
E.
Apotex process: ||||||||||||||||||||||
[386]
Dr. Laird agreed that the process outlined in the batch record would not infringe, but he alleges that the process is fabricated. As such, Lilly raise the two presumptions, while Apotex denies their applicability. I have found the presumptions not to apply, and Lilly have not met their burden to establish infringement.
[387]
Dr. Laird, Lilly’s expert, confirmed that the process outlined in Apotex’s ANDS, which was produced by Lilly as business records, does not infringe. Dr. Laird did not have the batch records for other processes and based his infringement analysis on the flow diagrams, ANDS, or open part of the drug master file (DMF) of those processes.
[388]
Dr. Laird, in his Infringement–Expert Report, confirms that the ANDS of the |||||||||||||||||||| process describe a PSR in which the |||||||||||||||||||||||||||||||||||||||||||||||||||| is reacted with |||||||||| using the |||||||||||||||||||||||||||||||||||||||||||||||||||||||| methodology, different from the 540 Patent process, which impacts Claims 1, 3–4, 12.
[389]
Regarding Claim 7, the ANDS indicates that after the ||||||||||||||||||||||||||||||, |||| is added to the ||||||||||||, |||||||| and |||| followed by the |||||||| with |||||||||| and |||||||||||||||||||||||||| and |||||||| with |||||||||| (Dr. Laird Infringement Report Schedule J Tab 2 at 3). There is, therefore, no purification by recrystallization with acetic acid, essential element of Claim 7d. There would be no infringement of Claims 7–10.
[390]
Lilly has not met their burden to establish that the |||||||||||||||||||||| infringes any of the asserted Claims.
F.
Conclusion on infringement
[391]
If I am wrong and the asserted Claims are valid, Lilly have established that the |||||||||| process infringes Claims 1, 3-4 of the 540 Patent.
VIII.
Election between damages and accounting of profits
[392]
If I were wrong on the invalidity of the asserted Claims, Claim 1 would be infringed and unless decided otherwise during the quantification phase, Lilly would be entitled to elect between damages and accounting of profit. It is generally the rule that the trial judge has complete discretion in deciding whether or not to grant this equitable remedy (Merck & Co v Apotex Inc, 2006 FCA 323), and the right to elect has been denied for a variety of reasons such as the delay in bringing forward an action for infringement, misconduct on the part of the patentee and good faith of an infringer (Cefaclor at paras 647–648).
IX.
Declaratory relief, injunction relief and/or delivery up.
[393]
The Court has discretion to issue declaratory injunction, and delivery up reliefs (Patent Act, s 57). Should the asserted Claims be valid, I would declare that Apotex infringed Claims 1, 3 and 4 of the Patent through the |||||||||| process and grant Lilly the reliefs sought.
X.
Sealing Order
[394]
The parties have fifteen days from the release of these confidential reasons to make submissions as to what should not be released to the public.
XI.
Costs
[395]
The parties made scarce representations as to costs, they have no asked for the Court to reserve nor for the opportunity to file submissions on this issue. Lilly asked for “costs of this action on a scale to be determined by this Court, including all applicable taxes and disbursements”
while Apotex asked that this action, as it relates to the 540 Patent, be dismissed with costs payable to Apotex.
[396]
As Lilly’s action relating to the 540 Patent is dismissed and Apotex’s counterclaim is granted, costs are payable to Apotex.
PUBLIC JUDGMENT in T-1632-16
THIS COURT’S JUDGMENT is that:
1. The infringement action against the Defendant relating to Canadian Patents Nos. 2,371,684 and 2,492,540 is dismissed.
2. Claims 1, 3-4 of Canadian Patent No. 2,492,540 are invalid for anticipation, and Claims 1, 3-4, 7-10, 12 of Canadian Patent No. 2,492,540 are invalid for obviousness.
3. Claim 10 (as it depends on Claim 9, as it in turn depends on Claims 3–6), and Claims 13–16 of Canadian Patent No. 2,371,684 are invalid for anticipation and obviousness.
4 Costs are granted in favor of the Defendant.
5 The parties have fifteen days from the release of the confidential reasons to make submissions on redactions before a public version is released.
6 Copy of the confidential reasons exposed in case T-1627-16 shall be placed on this file.
“Martine St-Louis”
Judge
ANNEX
As the patents in suit were issued after October 1, 1989, the provisions of the current Patent Act apply. The relevant sections of the Patent Act provide as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ANNEX II
A method of preparing a desired cis-diastereomer of a tetrahydro-β-carboline having a formula
comprising the steps of:
a) providing a tryptophan esterified using an alcohol having a formula R2OH wherein R2 is selected from methyl, ethyl, isopropyl, n-propyl, n-butyl, sec-butyl, t-butyl, and mixtures thereof;
b) reacting, the tryptophan ester of step (a) with an aldehyde having a formula R1CHO wherein R1 is piperonyl, to provide the desired diastereomer and an undesired diastereomer wherein the reaction is performed in a solvent in which the desired diastereomer is insoluble at reflux temperature or lower and the undesired diastereomer is soluble at reflux termperature or lower, and
c) separating the insoluble desired diastereomer from the soluble undesired diastereomer.
The method of claim 1 wherein the alcohol R2OH is methanol.
The method of claim 1 wherein the tryptophan is D-trytophan.
A method of preparing a compound having a formula
comprising the steps of:
a) providing a desired diastereomer of a tetrahydro-β-carboline by the method of claim 1;
b) reacting the tetrahydro-β-carboline with chloroacetyl chloride to provide an N-substituted tetrahydro-β-carboline;
c) reacting the N-substituted tetrahydro-β-carboline with an amine having a structure R3NH2, wherein R3 is C1-6aIkyl or hydro; and
d) purifying the compound by recrystallization from glacial acetic acid.
- The method of claim 7 wherein the amine is selected from the group consisting of ammonia, methylamine, ethylamine, propylamine, isopropylamine, butyl amine, and sec-butyl amine.
- The method of claim 7 wherein the amine is methylamine.
- The method of claim 7 wherein R3 is methyl.
12. A method of preparing a compound having a structural formula:
comprising the steps of:
a) esterifying D tryptophan in methanol and thionyl chloride to provide D-tryptophan methyl ester hydrochloride,
b) reacting the D-tryptophan methyl ester hydrochloride with piperonal in refluxing isopropyl alcohol to provide cis-1-(1,3-benzodioxol-5-y1)-2,3,4,9-tetrahydro 1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester;
c) reacting the product of step (b) with chloroacetyl chloride and triethylamine to provide cis-1-(1,3-benzodioxol-5-y1)-2,3,4,9-tetrahydro 1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester; and
d) reacting the product of step (c) with methylamine to provide the compound.
FEDERAL COURT
SOLICITORS OF RECORD
| DOCKET:
|
T-1632-16
|
| STYLE OF CAUSE:
|
ELI LILLY CANADA INC., ELI LILLY AND COMPANY, LILLY DELCARIBE INC, LILLY, S.A. AND ICOS CORPORATION INC. and APOTEX INC.
|
| PLACE OF HEARING:
|
Ottawa, Ontario
|
| DATE OF HEARING:
|
from DECEMBER 4, 2019 to february 7, 2020
|
| JUDGMENT AND reasons:
|
THE honorable justice martine st-louis
|
| DATED:
|
September 10, 2020
|
APPEARANCES:
| Jamie Mills
Adrian Howard
Chantal Saunders
Beverley Moore
David Schnittker
David Chapman
|
PLAINTIFFS/DEFENDANTS BY COUNTERCLAIM
|
| Andrew Brodkin
Jordan Scopa
Jaclyn Tilak
Benjamin Hackett
|
DEFENDANT/PLAINTIFF BY COUNTERCLAIM
|
SOLICITORS OF RECORD:
| Borden Ladner Gervais
Ottawa (Ontario)
|
PLAINTIFFS/DEFENDANTS BY COUNTER CLAIM
|
| Goodmans LLP
Toronto (Ontario)
|
DEFENDANT/PLAINTIFF BY COUNTERCLAIM
|